KIR-HLA Functional Repertoire Influences Trastuzumab Efficiency in Patients With HER2-Positive Breast Cancer
- PMID: 35095867
- PMCID: PMC8790064
- DOI: 10.3389/fimmu.2021.791958
KIR-HLA Functional Repertoire Influences Trastuzumab Efficiency in Patients With HER2-Positive Breast Cancer
Abstract
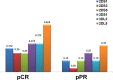
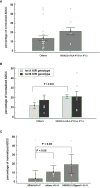
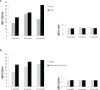
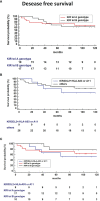
Trastuzumab induced a high rate of pathological Complete Response (pCR) in patients affected by locally advanced HER2-positive Breast Cancer (HER2-BC), by exploiting immune-mediated mechanisms as Antibody-Dependent Cell Cytotoxicity (ADCC) involving Natural Killer (NK) cells. Host's immune genetics could influence the response to therapy, through the expression of variants that characterize NK receptors involved in ADCC effectiveness. Killer cell immunoglobin-like receptors (KIRs) modulate NK cell activity through their binding to class-I Human Leukocyte Antigens (HLA). The impact of the KIR/HLA repertoire in HER2-BC is under study. We characterized KIR genotypes of 36 patients with locally advanced HER2-BC treated with neoadjuvant chemotherapy including trastuzumab. We monitored pCR achievement before surgery and Disease-Free Survival (DFS) and Overall Survival (OS) after adjuvant therapy. HLA, and Fc gamma receptor IIIa (FcγR3A) and IIa (FcγR2A) were genotyped through targeted PCR and Sanger sequencing in 35/36 patients. The KIR-HLA combinations were then described as functional haplotypes and divided in two main categories as inhibitory tel A and stimulatory tel B. Trastuzumab-dependent ADCC activity was monitored with an in vitro assay using a HER2-BC model and patients' NK cells.We observed a higher frequency of KIR activators in patients who achieved a pCR compared to partial responders. During the study of functional haplotypes, individuals carrying a tel B haplotype showed greater ADCC efficiency than tel A cases. In subjects with the tel A haplotype the presence of the favorite V allele in FcγR3A receptor improved their low ADCC levels. Regardless of the haplotypes detected, the presence of KIR3DL2/HLA-A03 or A11 was always associated with the FcγR3A V allele, and therefore correlated with greater ADCC efficiency. However, this particular KIR receptor appeared to harm DFS and OS. Indeed, patients with tel B haplotype without KIR3DL2/HLA-A03 or A11 showed a better outcome. Our data, although preliminary, suggested a potential predictive role for KIR haplotype tel B, in identifying patients who achieve a pCR after neoadjuvant treatment with trastuzumab, and supported a negative prognostic impact of KIR3DL2/HLA-A03 or A11 in the adjuvant setting.
Trial registration: ClinicalTrials.gov NCT02307227.
Keywords: ADCC; HLA; KIR; breast cancer; trastuzumab.
Copyright © 2022 Muraro, De Zorzi, Miolo, Lombardi, Scalone, Spazzapan, Massarut, Perin, Dolcetti, Steffan and De Re.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The editor PL declared a past co-authorship with one of the authors VR at the time of review.
Figures



References
-
- Gianni L, Pienkowski T, Im Y-H, Tseng L-M, Liu M-C, Lluch A, et al. . 5-Year Analysis of Neoadjuvant Pertuzumab and Trastuzumab in Patients With Locally Advanced, Inflammatory, or Early-Stage HER2-Positive Breast Cancer (NeoSphere): A Multicentre, Open-Label, Phase 2 Randomised Trial. Lancet Oncol (2016) 17:791–800. doi: 10.1016/S1470-2045(16)00163-7 - DOI - PubMed
-
- Gianni L, Eiermann W, Semiglazov V, Lluch A, Tjulandin S, Zambetti M, et al. . Neoadjuvant and Adjuvant Trastuzumab in Patients With HER2-Positive Locally Advanced Breast Cancer (NOAH): Follow-Up of a Randomised Controlled Superiority Trial With a Parallel HER2-Negative Cohort. Lancet Oncol (2014) 15:640–7. doi: 10.1016/S1470-2045(14)70080-4 - DOI - PubMed
-
- Muraro E, Comaro E, Talamini R, Turchet E, Miolo G, Scalone S, et al. . Improved Natural Killer Cell Activity and Retained Anti-Tumor CD8(+) T Cell Responses Contribute to the Induction of a Pathological Complete Response in HER2-Positive Breast Cancer Patients Undergoing Neoadjuvant Chemotherapy. J Transl Med (2015) 13:204. doi: 10.1186/s12967-015-0567-0 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

