Disease-Modifying Drug Uptake and Health Service Use in the Ageing MS Population
- PMID: 35095869
- PMCID: PMC8792855
- DOI: 10.3389/fimmu.2021.794075
Disease-Modifying Drug Uptake and Health Service Use in the Ageing MS Population
Abstract
Background: Evidence regarding the efficacy or effectiveness of the disease-modifying drugs (DMDs) in the older multiple sclerosis (MS) population is scarce. This has contributed to a lack of evidence-based treatment recommendations for the ageing MS population in practice guidelines. We examined the relationship between age (<55 and ≥55 years), DMD exposure and health service use in the MS population.
Methods: We conducted a population-based observational study using linked administrative health data from British Columbia, Canada. We selected all persons with MS and followed from the most recent of their first MS or demyelinating event, 18th birthday or 01-January-1996 (index date) until the earliest of emigration, death or 31-December-2017 (study end). We assessed DMD exposure status over time, initially as any versus no DMD, then by generation (first or second) and finally by each individual DMD. Age-specific analyses were conducted with all-cause hospitalizations and number of physician visits assessed using proportional means model and negative binomial regression with generalized estimating equations.
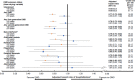
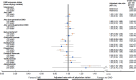
Results: We included 19,360 persons with MS (72% were women); 10,741/19,360 (56%) had ever reached their 55th birthday. Person-years of follow-up whilst aged <55 was 132,283, and 93,594 whilst aged ≥55. Any DMD, versus no DMD in the <55-year-olds was associated with a 23% lower hazard of hospitalization (adjusted hazard ratio, aHR0.77; 95%CI 0.72-0.82), but not in the ≥55-year-olds (aHR0.95; 95%CI 0.87-1.04). Similar patterns were observed for the first and second generation DMDs. Exposure to any (versus no) DMD was not associated with rates of physician visits in either age group (<55 years: adjusted rate ratio, aRR1.02; 95%CI 1.00-1.04 and ≥55 years: aRR1.00; 95%CI 0.96-1.03), but variation in aRR was observed across the individual DMDs.
Conclusion: Our study showed beneficial effects of the DMDs used to treat MS on hospitalizations for those aged <55 at the time of exposure. In contrast, for individuals ≥55 years of age exposed to a DMD, the hazard of hospitalization was not significantly lowered. Our study contributes to the broader understanding of the potential benefits and risks of DMD use in the ageing MS population.
Keywords: ageing; cohort studies; disease-modifying drugs; health services; hospitalization; multiple sclerosis; physician services.
Copyright © 2022 Ng, Graf, Zhu, Kingwell, Aktas, Albrecht, Hartung, Meuth, Evans, Fisk, Marrie, Zhao and Tremlett.
Conflict of interest statement
HN receives funding from the Multiple Sclerosis Society of Canada’s endMS Postdoctoral Fellowship, and the Michael Smith Foundation for Health Research Trainee Award. During the past year, HSN has received funding from the Canadian Institutes of Health Research (CIHR) Drug Safety and Effectiveness Cross-Disciplinary Training Program. JG has received in the last 3 years travel/meeting/accommodation reimbursements from Merck Serono, Sanofi-Genzyme, Grifols, and receives a Research Fellowship from the Deutsche Forschungsgemeinschaft (project number 438899010, GZ: GR 5665/1-1). OA reports research funding from: German Research Foundation (DFG), German Ministry of Science (BMBF), German National MS Society (DMSG-LV), Biogen and Novartis; personal fees from Alexion, Biogen, EMD, Novartis, Roche, and Teva. PA reports research funding and personal fees from: European Fund for Regional Development (EFRE), Allergan, Celgene, Biogen, Ipsen, Merck, Merz, Roche; personal fees from Janssen Cilag, Lilly, and Teva. H-PH received outside this work, with approval of the Rector of Heinrich-Heine University and the CEO of University of Düsseldorf Hospital honoraria for consulting, serving on steering committees and speaking from Alexion, Bayer Healthcare, Biogen, Celgene BMS, Geneuro, LFB, Medimmune, Merck Serono, Novartis, Octapharma, Roche, Sanofi Genzyme, TG Therapeutics and VielaBio. SM received honoraria for lecturing and travel expenses for attending meetings from Almirall, Amicus Therapeutics Germany, Bayer Health Care, Biogen, Celgene, Diamed, Genzyme, MedDay Pharmaceuticals, Merck Serono, Novartis, Novo Nordisk, ONO Pharma, Roche, Sanofi-Aventis, Chugai Pharma, QuintilesIMS, and Teva and his research is funded by the German Ministry for Education and Research (BMBF), Deutsche Forschungsgemeinschaft (DFG), Else Kröner Fresenius Foundation, German Academic Exchange Service, Hertie Foundation, Interdisciplinary Center for Clinical Studies (IZKF) Muenster, German Foundation Neurology, and by Almirall, Amicus Therapeutics Germany, Biogen, Diamed, Fresenius Medical Care, Genzyme, Merck Serono, Novartis, ONO Pharma, Roche, and Teva. CE receives funding from CIHR. JF receives research funding from: CIHR, Multiple Sclerosis Society of Canada, Crohn’s and Colitis Canada, Research Nova Scotia; consultation and distribution royalties from MAPI Research Trust. RM receives research funding from: CIHR, Research Manitoba, Multiple Sclerosis Society of Canada, Multiple Sclerosis Scientific Foundation, Crohn’s and Colitis Canada, National Multiple Sclerosis Society, CMSC and the US Department of Defense, and is a co-investigator on studies receiving funding from Biogen Idec and Roche Canada. HT is the Canada Research Chair for Neuroepidemiology and Multiple Sclerosis. Current research support received from the National Multiple Sclerosis Society, the Canadian Institutes of Health Research, the Multiple Sclerosis Society of Canada and the Multiple Sclerosis Scientific Research Foundation. In addition, in the last five years, has received research support from the UK MS Trust; travel expenses to present at CME conferences from the Consortium of MS Centres (2018), the National MS Society (2016, 2018), ECTRIMS/ACTRIMS (2015, 2016, 2017, 2018, 2019, 2020), American Academy of Neurology (2015, 2016, 2019). Speaker honoraria are either declined or donated to an MS charity or to an unrestricted grant for use by HT’s research group. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Rae-Grant A, Day GS, Marrie RA, Rabinstein A, Cree BAC, Gronseth GS, et al. . Practice Guideline Recommendations Summary: Disease-Modifying Therapies for Adults With Multiple Sclerosis. Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology (2018) 90:777–88. doi: 10.1212/WNL.0000000000005347 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

