A Personalized Rituximab Retreatment Approach Based on Clinical and B-Cell Biomarkers in ANCA-Associated Vasculitis
- PMID: 35095887
- PMCID: PMC8789753
- DOI: 10.3389/fimmu.2021.803175
A Personalized Rituximab Retreatment Approach Based on Clinical and B-Cell Biomarkers in ANCA-Associated Vasculitis
Abstract
Background: Time to relapse after rituximab for the treatment of antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) is variable, and optimal retreatment strategy has remained unclear. In AAV following rituximab induction, the study objective was to evaluate clinical and B-cell predictors of relapse in order to develop a retreatment algorithm.
Methods: A retrospective observational study was conducted in 70 rituximab-treated ANCA-associated vasculitis patients followed up for over 10 years. Complete response (CR) was defined as Birmingham Vasculitis Activity Score v3.0 = 0. Retreatment was given on clinical relapse, defined as new features or worsening of persistent disease (not by biomarker status). Peripheral B-cell subsets were measured using highly sensitive flow cytometry. Predictors were tested using multivariable Cox regression.
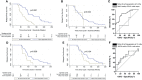
Results: Median time to retreatment for cycles 1-5 were 84, 73, 67, 60, and 73 weeks. Over 467 patient-years follow-up, 158 relapses occurred in 60 patients; 16 (in 15 patients) were major (renal = 7, neurological = 4, ENT = 3, and respiratory = 2). The major-relapse rate was 3.4/100 patient-years. In multivariable analysis, concomitant immunosuppressant [HR, 0.48 (95% CI, 0.24-0.94)], achieving CR [0.24 (0.12-0.50)], and naïve B-cell repopulation at 6 months [0.43 (0.22-0.84)] were associated with longer time to relapse. Personalized retreatment using these three predictors in this cohort would have avoided an unnecessary fixed retreatment in 24% of patients. Area under the receiver operating characteristic for prediction of time to relapse was greater if guided by naïve B-cell repopulation than if previously evaluated ANCA and/or CD19+ cells return at 6 months had been used, 0.82 and 0.53, respectively.
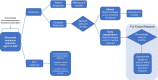
Conclusion: Our findings suggest that all patients should be coprescribed oral immunosuppressant. Those with incomplete response or with absent naïve B cells should be retreated at 6 months. Patients with complete response and naïve repopulation should not receive fixed retreatment. This algorithm could reduce unnecessary retreatment and warrant investigation in clinical trials.
Keywords: B cell; cyclophosphamide; immunoglobulin; rituximab; vasculitis.
Copyright © 2022 Arnold, Vital, Dass, Aslam, Rawstron, Savic, Emery and Md Yusof.
Conflict of interest statement
SD has received honoraria from Roche and GSK. SS has received honoraria from Novartis, Swedish Orphan Biovitrum (SOBI), and Sire and grant support from Novartis, Swedish Orphan Biovitrum, Octapharma, and CSL Behring. EV has received honoraria and research grant support from Roche, GSK, and AstraZeneca. PE has received consultant fees from BMS, Abbott, Pfizer, MSD, Novartis, Roche, and UCB. He has received research grants paid to his employer from Abbott, BMS, Pfizer, MSD, and Roche. MYMY has received consultancy fees from Aurinia Pharmaceuticals. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Repeat cycles of rituximab on clinical relapse in ANCA-associated vasculitis: identifying B cell biomarkers for relapse to guide retreatment decisions.Ann Rheum Dis. 2015 Sep;74(9):1734-8. doi: 10.1136/annrheumdis-2014-206496. Epub 2015 Apr 8. Ann Rheum Dis. 2015. PMID: 25854586
-
Highly Sensitive Flow Cytometric Detection of Residual B-Cells After Rituximab in Anti-Neutrophil Cytoplasmic Antibodies-Associated Vasculitis Patients.Front Immunol. 2020 Dec 15;11:566732. doi: 10.3389/fimmu.2020.566732. eCollection 2020. Front Immunol. 2020. PMID: 33384685 Free PMC article.
-
Comparison of individually tailored versus fixed-schedule rituximab regimen to maintain ANCA-associated vasculitis remission: results of a multicentre, randomised controlled, phase III trial (MAINRITSAN2).Ann Rheum Dis. 2018 Aug;77(8):1143-1149. doi: 10.1136/annrheumdis-2017-212878. Epub 2018 Apr 25. Ann Rheum Dis. 2018. PMID: 29695500 Clinical Trial.
-
Current and emerging treatment options for ANCA-associated vasculitis: potential role of belimumab and other BAFF/APRIL targeting agents.Drug Des Devel Ther. 2015 Jan 7;9:333-47. doi: 10.2147/DDDT.S67264. eCollection 2015. Drug Des Devel Ther. 2015. PMID: 25609919 Free PMC article. Review.
-
Therapy and prognosis of ANCA-associated vasculitis from the clinical nephrologist's perspective.Int Urol Nephrol. 2017 Jan;49(1):91-102. doi: 10.1007/s11255-016-1419-4. Epub 2016 Sep 26. Int Urol Nephrol. 2017. PMID: 27671907 Review.
Cited by
-
Systematic literature review informing the 2022 update of the EULAR recommendations for the management of ANCA-associated vasculitis (AAV): part 1-treatment of granulomatosis with polyangiitis and microscopic polyangiitis.RMD Open. 2023 Jul;9(3):e003082. doi: 10.1136/rmdopen-2023-003082. RMD Open. 2023. PMID: 37479496 Free PMC article.
-
Predicting Sustained Clinical Response to Rituximab in Moderate to Severe Systemic Manifestations of Primary Sjögren Syndrome.ACR Open Rheumatol. 2022 Aug;4(8):689-699. doi: 10.1002/acr2.11466. Epub 2022 Jun 5. ACR Open Rheumatol. 2022. PMID: 35666029 Free PMC article.
-
How We Treat ANCA-Associated Vasculitis: A Focus on the Maintenance Therapy.J Clin Med. 2025 Jan 2;14(1):208. doi: 10.3390/jcm14010208. J Clin Med. 2025. PMID: 39797292 Free PMC article. Review.
-
Features of BAFF and APRIL receptors on circulating B cells in antineutrophil cytoplasmic antibody-associated vasculitis.Clin Exp Immunol. 2023 Jul 5;213(1):125-137. doi: 10.1093/cei/uxad024. Clin Exp Immunol. 2023. PMID: 36794867 Free PMC article.
-
Treating Patients With ANCA-Associated Vasculitis and Very Severe Renal Injury With an Intensified B Cell Depletion Therapy: Comparison With a Control Cohort Receiving a Conventional Therapy.Front Immunol. 2022 Mar 24;13:777134. doi: 10.3389/fimmu.2022.777134. eCollection 2022. Front Immunol. 2022. PMID: 35401565 Free PMC article.
References
-
- Cartin-Ceba R, Golbin JM, Keogh KA, Peikert T, Sanchez-Menendez M, Ytterberg SR, et al. . Rituximab for Remission Induction and Maintenance in Refractory Granulomatosis With Polyangiitis (Wegener's): Ten-Year Experience at a Single Center. Arthritis Rheum (2012) 64(11):3770–8. doi: 10.1002/art.34584 - DOI - PubMed
-
- Md Yusof MY, Vital EM, Das S, Dass S, Arumugakani G, Savic S, et al. . Repeat Cycles of Rituximab on Clinical Relapse in ANCA-Associated Vasculitis: Identifying B Cell Biomarkers for Relapse to Guide Retreatment Decisions. Ann Rheum Dis (2015) 74(9):1734–8. doi: 10.1136/annrheumdis-2014-206496 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

