Evaluation of Medication Package Inserts in Ethiopia
- PMID: 35096074
- PMCID: PMC8791750
- DOI: 10.1155/2022/8299218
Evaluation of Medication Package Inserts in Ethiopia
Abstract
Background: Patients require accurate and reliable information to help them use their medications safely and effectively. Inadequate patient knowledge may contribute to medication nonadherence which could negatively affect treatment outcomes. The purpose of this study was to evaluate the presentation and completeness of medication package inserts (MPIs) which are available in the Ethiopian market.
Methods: A cross-sectional document review was performed in February and March of 2019. All MPIs which were authorized by EFDA to sell in the Ethiopian market and available during the data collection period were considered.
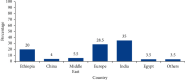
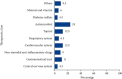
Results: The mean overall completeness score of 200 MPIs was 18.39 ± 4.30. Of the 200 MPIs, only 20% were from domestic pharmaceutical companies. Antimicrobials represented 24% of the total MPIs. Topical preparations, cardiovascular drugs, gastrointestinal drugs, and nonsteroidal anti-inflammatory drugs, accounted for 12.5%,12.5%, 11%, and 9% of the MPIs, respectively. The majority of the MPIs presented information about the drug's use during pregnancy and lactation, 77.0% and 74.0%, respectively. However, only half of the MPIs, 49.5%, gave information about special warnings and precautions. Only a few of the MPIs provided information about instructions to convert tablets or capsules into liquid forms and the possibility of tablet splitting, 4.8% and 8.7%, respectively. Furthermore, only 1.0% had local language translation.
Conclusion: The MPIs available in Ethiopia provide inadequate information including about the safety of drug products and local language translation. Regulatory authorities should implement stringent regulations to ensure the provision of vital information which extends beyond checking the mere presence of an MPI. They should also act to the possible standardization of MPIs.
Copyright © 2022 Haftom Gebregergs Hailu et al.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Jain A., Jindal A., Bansal N., Arora H. Comparison of drug information in package inserts with standard medical textbook of pharmacology. International Journal of Basic & Clinical Pharmacology . 2018;7(5):p. 1002. doi: 10.18203/2319-2003.ijbcp20181650. - DOI
-
- Joseph B. N., Asiegbu U. O., Aya B. M., et al. Usability of Medicine Package Inserts for Chronic Diseases: A Survey of the Pharmaceutical Market in Jos, Nigeria. Journal of Pharmaceutical Research International . 2017;7(4):1–10.
-
- Deepak K., Gaur A. Scope of improvement of patient information leaflets in randomly selected therapeutic classes of drugs. International Journal of Pharma Sciences and Research . 2018;9:46–50.
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous