The HeyL-Aromatase Axis Promotes Cancer Stem Cell Properties by Endogenous Estrogen-Induced Autophagy in Castration-Resistant Prostate Cancer
- PMID: 35096586
- PMCID: PMC8789881
- DOI: 10.3389/fonc.2021.787953
The HeyL-Aromatase Axis Promotes Cancer Stem Cell Properties by Endogenous Estrogen-Induced Autophagy in Castration-Resistant Prostate Cancer
Abstract
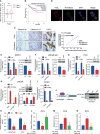
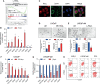
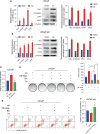
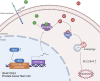
Treatment of patients with castration-resistant prostate cancer (CRPC) remains a major clinical challenge. We previously showed that estrogenic effects contribute to CRPC progression and are primarily caused by the increased endogenous estradiol produced via highly expressed aromatase. However, the mechanism of aromatase upregulation and its role in CRPC are poorly described. In this study, we report that HeyL is aberrantly upregulated in CRPC tissues, and its expression is positively correlated with aromatase levels. HeyL overexpression increased endogenous estradiol levels and estrogen receptor-α (ERα) transcriptional activity by upregulating CYP19A1 expression, which encodes aromatase, enhancing prostate cancer stem cell (PCSC) properties in PC3 cells. Mechanistically, HeyL bound to the CYP19A1 promoter and activated its transcription. HeyL overexpression significantly promoted bicalutamide resistance in LNCaP cells, which was reversed by the aromatase inhibitor letrozole. In PC3 cells, the HeyL-aromatase axis promoted the PCSC phenotype by upregulating autophagy-related genes, while the autophagy inhibitor chloroquine (CQ) suppressed the aromatase-induced PCSC phenotype. The activated HeyL-aromatase axis promoted PCSC autophagy via ERα-mediated estrogenic effects. Taken together, our results indicated that the HeyL-aromatase axis could increase endogenous estradiol levels and activate ERα to suppress PCSC apoptosis by promoting autophagy, which enhances the understanding of how endogenous estrogenic effects influence CRPC development.
Keywords: CRPC; HeyL; aromatase; autophagy; prostate cancer stem cell.
Copyright © 2022 Lin, Cao, Du, Yang, Shen, Wang, Klocker, Shi and Zhang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Aromatase-induced endogenous estrogen promotes tumour metastasis through estrogen receptor-α/matrix metalloproteinase 12 axis activation in castration-resistant prostate cancer.Cancer Lett. 2019 Dec 28;467:72-84. doi: 10.1016/j.canlet.2019.09.001. Epub 2019 Sep 6. Cancer Lett. 2019. PMID: 31499120
-
CYP1B1-catalyzed 4-OHE2 promotes the castration resistance of prostate cancer stem cells by estrogen receptor α-mediated IL6 activation.Cell Commun Signal. 2022 Mar 15;20(1):31. doi: 10.1186/s12964-021-00807-x. Cell Commun Signal. 2022. PMID: 35292057 Free PMC article.
-
NPRL2 enhances autophagy and the resistance to Everolimus in castration-resistant prostate cancer.Prostate. 2019 Jan;79(1):44-53. doi: 10.1002/pros.23709. Epub 2018 Sep 3. Prostate. 2019. PMID: 30178500
-
Hypoxia induced cancer stem cell enrichment promotes resistance to androgen deprivation therapy in prostate cancer.Steroids. 2019 Dec;152:108497. doi: 10.1016/j.steroids.2019.108497. Epub 2019 Sep 12. Steroids. 2019. PMID: 31521707 Review.
-
Estrogen metabolites and breast cancer.Steroids. 2015 Jul;99(Pt A):61-6. doi: 10.1016/j.steroids.2014.08.003. Epub 2014 Aug 26. Steroids. 2015. PMID: 25168343 Review.
Cited by
-
The dual role of autophagy in cancer stem cells: implications for tumor progression and therapy resistance.J Transl Med. 2025 May 25;23(1):583. doi: 10.1186/s12967-025-06595-z. J Transl Med. 2025. PMID: 40414839 Free PMC article. Review.
-
Development of a 5-mRNAsi-related gene signature to predict the prognosis of colon adenocarcinoma.PeerJ. 2023 Nov 24;11:e16477. doi: 10.7717/peerj.16477. eCollection 2023. PeerJ. 2023. PMID: 38025763 Free PMC article.
-
HEYL-mediated activation of LAMA3 influences radiotherapy response in esophageal cancer.Esophagus. 2025 Aug 20. doi: 10.1007/s10388-025-01147-2. Online ahead of print. Esophagus. 2025. PMID: 40833662
-
Prostate Cancer Progression Modeling Provides Insight into Dynamic Molecular Changes Associated with Progressive Disease States.Cancer Res Commun. 2024 Oct 1;4(10):2783-2798. doi: 10.1158/2767-9764.CRC-24-0210. Cancer Res Commun. 2024. PMID: 39347576 Free PMC article.
-
Prostate cancer induced bone pain: pathobiology, current treatments and pain responses from recent clinical trials.Discov Oncol. 2022 Oct 18;13(1):108. doi: 10.1007/s12672-022-00569-z. Discov Oncol. 2022. PMID: 36258057 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources

