Optimizing Patient Selection for Irreversible Electroporation of Locally Advanced Pancreatic Cancer: Analyses of Survival
- PMID: 35096621
- PMCID: PMC8793779
- DOI: 10.3389/fonc.2021.817220
Optimizing Patient Selection for Irreversible Electroporation of Locally Advanced Pancreatic Cancer: Analyses of Survival
Abstract
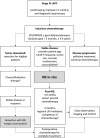
Background: Irreversible electroporation (IRE) has emerged as a viable consolidative therapy after induction chemotherapy, in which this combination has improved overall survival of locally advanced pancreatic cancer (LAPC). Optimal timing and patient selection for irreversible electroporation remains a clinically unmet need. The aim of this study was to investigate preoperative factors that may assist in predicting progression-free and overall survival following IRE.
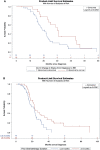
Methods: A multi-institutional, prospectively maintained database was reviewed for patients with LAPC treated with induction chemotherapy followed by open-technique irreversible electroporation from 7/2015-5/2019. RECIST 1.1 criteria were used to assess tumor response and radiological progression. Overall survival (OS) and progression-free survival (PFS) were recorded. Survival analyses were performed using Kaplan Meier and Cox multivariable regression analyses.
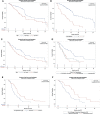
Results: 187 LAPC patients (median age 62 years range, 21 - 91, 65% men, 35% women) were treated with IRE. Median PFS was 21.7 months and median OS from diagnosis was 25.5 months. On multivariable analysis, age ≤ 61 (HR 0.41, 95%CI 0.21-0.78, p<0.008) and no prior radiation (HR 0.49, 95%CI 0.26-0.94, p=0.03) were positive predictors of OS after IRE. Age ≤ 61(HR 0.53, 95%CI, 0.28-.99, p=0.046) and FOLFIRINOX followed by gemcitabine/abraxane induction chemotherapy (HR 0.37,95%CI 0.15-0.89, p=0.027) predicted prolonged PFS after IRE. Abnormal CA19-9 values at the time of surgery negatively impacted both OS (HR 2.46, 95%CI 1.28-4.72, p<0.007) and PFS (HR 2.192, 95%CI 1.143-4.201, p=0.018) following IRE.
Conclusions: Age, CA 19-9 response, avoidance of pre-IRE radiation, and FOLFIRINOX plus gemcitabine/abraxane induction chemotherapy are prominent factors to consider when referring or selecting LAPC patients to undergo IRE.
Keywords: irreversible electroporation (IRE); locally advanced pancreatic cancer; overall survival; patient selection; progression free survival; recurrence.
Copyright © 2022 Woeste, Wilson, Kruse, Weiss, Christein, White and Martin.
Conflict of interest statement
RM II, MD, PhD, FACS is an educational consultant for AngioDynamics, Inc. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Pancreatic Cancer Action Network . Pancreatic Cancer Survival Rates (2020). Available at: https://www.pancan.org/facing-pancreatic-cancer/about-pancreatic-cancer/.
-
- Michelakos T, Pergolini I, Castillo CF, Honselmann KC, Cai L, Deshpande V, et al. . Predictors of Resectability and Survival in Patients With Borderline and Locally Advanced Pancreatic Cancer Who Underwent Neoadjuvant Treatment With FOLFIRINOX. Ann Surg (2019) 269(4):733–40. doi: 10.1097/SLA.0000000000002600 - DOI - PubMed
LinkOut - more resources
Full Text Sources

