Long Non-Coding RNA CD27-AS1-208 Facilitates Melanoma Progression by Activating STAT3 Pathway
- PMID: 35096622
- PMCID: PMC8791859
- DOI: 10.3389/fonc.2021.818178
Long Non-Coding RNA CD27-AS1-208 Facilitates Melanoma Progression by Activating STAT3 Pathway
Abstract
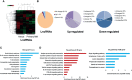
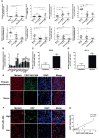
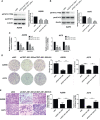
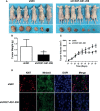
Melanoma is the most lethal skin cancer that originates from epidermal melanocytes. Recently, long non-coding RNAs (lncRNAs) are emerging as critical regulators of cancer pathogenesis and potential therapeutic targets. However, the expression profile of lncRNAs and their role in melanoma progression have not been thoroughly investigated. Herein, we firstly obtained the expression profile of lncRNAs in primary melanomas using microarray analysis and unveiled the differentially-expressed lncRNAs compared with nevus. Subsequently, a series of bioinformatics analysis showed the great involvement of dysregulated lncRNAs in melanoma biology and immune response. Further, we identified lncRNA CD27-AS1-208 as a novel nuclear-localized factor with prominent facilitative role in melanoma cell proliferation, invasion and migration. Mechanistically, CD27-AS1-208 could directly interact with STAT3 and contribute to melanoma progression in a STAT3-dependent manner. Ultimately, the role of CD27-AS1-208 in melanoma progression in vivo was also investigated. Collectively, the present study offers us a new horizon to better understand the role of lncRNAs in melanoma pathogenesis and demonstrates that CD27-AS1-208 up-regulation contributes to melanoma progression by activating STAT3 pathway. Targeting CD27-AS1-208 in melanoma cells can be exploited as a potential therapeutic approach that needs forward validation in clinical trials in the future.
Keywords: CD27-AS1-208; LncRNAs; STAT3; melanoma; therapeutic targets.
Copyright © 2022 Ma, Shi, Guo, Xu, Yi, Yang, Zhang, Liu, Liu, Yue, Zhao, Gao, Guo and Li.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

