Diagnostic Approach to Macrocephaly in Children
- PMID: 35096710
- PMCID: PMC8795981
- DOI: 10.3389/fped.2021.794069
Diagnostic Approach to Macrocephaly in Children
Abstract
Macrocephaly affects up to 5% of the pediatric population and is defined as an abnormally large head with an occipitofrontal circumference (OFC) >2 standard deviations (SD) above the mean for a given age and sex. Taking into account that about 2-3% of the healthy population has an OFC between 2 and 3 SD, macrocephaly is considered as "clinically relevant" when OFC is above 3 SD. This implies the urgent need for a diagnostic workflow to use in the clinical setting to dissect the several causes of increased OFC, from the benign form of familial macrocephaly and the Benign enlargement of subarachnoid spaces (BESS) to many pathological conditions, including genetic disorders. Moreover, macrocephaly should be differentiated by megalencephaly (MEG), which refers exclusively to brain overgrowth, exceeding twice the SD (3SD-"clinically relevant" megalencephaly). While macrocephaly can be isolated and benign or may be the first indication of an underlying congenital, genetic, or acquired disorder, megalencephaly is most likely due to a genetic cause. Apart from the head size evaluation, a detailed family and personal history, neuroimaging, and a careful clinical evaluation are crucial to reach the correct diagnosis. In this review, we seek to underline the clinical aspects of macrocephaly and megalencephaly, emphasizing the main differential diagnosis with a major focus on common genetic disorders. We thus provide a clinico-radiological algorithm to guide pediatricians in the assessment of children with macrocephaly.
Keywords: brain MRI; genetic diagnosis; head circumference; macrocephaly; megalencephaly.
Copyright © 2022 Accogli, Geraldo, Piccolo, Riva, Scala, Balagura, Salpietro, Madia, Maghnie, Zara, Striano, Tortora, Severino and Capra.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
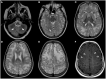
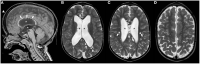
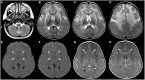
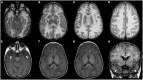
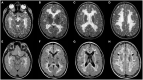
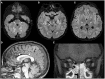
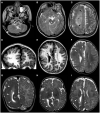
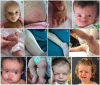
Figures





References
-
- Nellhaus G. Head circumference from birth to eighteen years. Pediatrics. (1968) 41:106–14. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

