Manipulating CD4+ T Cell Pathways to Prevent Preeclampsia
- PMID: 35096797
- PMCID: PMC8789650
- DOI: 10.3389/fbioe.2021.811417
Manipulating CD4+ T Cell Pathways to Prevent Preeclampsia
Abstract
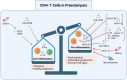
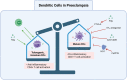
Preeclampsia (PreE) is a placental disorder characterized by hypertension (HTN), proteinuria, and oxidative stress. Individuals with PreE and their children are at an increased risk of serious short- and long-term complications, such as cardiovascular disease, end-organ failure, HTN, neurodevelopmental disorders, and more. Currently, delivery is the only cure for PreE, which remains a leading cause of morbidity and mortality among pregnant individuals and neonates. There is evidence that an imbalance favoring a pro-inflammatory CD4+ T cell milieu is associated with the inadequate spiral artery remodeling and subsequent oxidative stress that prime PreE's clinical symptoms. Immunomodulatory therapies targeting CD4+ T cell mechanisms have been investigated for other immune-mediated inflammatory diseases, and the application of these prevention tactics to PreE is promising, as we review here. These immunomodulatory therapies may, among other things, decrease tumor necrosis factor alpha (TNF-α), cytolytic natural killer cells, reduce pro-inflammatory cytokine production [e.g. interleukin (IL)-17 and IL-6], stimulate regulatory T cells (Tregs), inhibit type 1 and 17 T helper cells, prevent inappropriate dendritic cell maturation, and induce anti-inflammatory cytokine action [e.g. IL-10, Interferon gamma (IFN-γ)]. We review therapies including neutralizing monoclonal antibodies against TNF-α, IL-17, IL-6, and CD28; statins; 17-hydroxyprogesterone caproate, a synthetic hormone; adoptive exogenous Treg therapy; and endothelin-1 pathway inhibitors. Rebalancing the maternal inflammatory milieu may allow for proper spiral artery invasion, placentation, and maternal tolerance of foreign fetal/paternal antigens, thereby combatting early PreE pathogenesis.
Keywords: CD4+ T cells; early pregnancy; preeclampsia; prevention; treatment.
Copyright © 2022 Murray, Gumusoglu, Santillan and Santillan.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

