The Central Role of Extracellular Vesicles in the Mechanisms of Thrombosis in COVID-19 Patients With Cancer and Therapeutic Strategies
- PMID: 35096822
- PMCID: PMC8790316
- DOI: 10.3389/fcell.2021.792335
The Central Role of Extracellular Vesicles in the Mechanisms of Thrombosis in COVID-19 Patients With Cancer and Therapeutic Strategies
Abstract
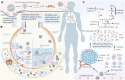
Cancer patients have increased SARS-CoV-2 susceptibility and are prone to developing severe COVID-19 infections. The incidence of venous thrombosis is approximately 20% in COVID-19 patients with cancer. It has been suggested that thrombus formation has been suggested to correlate with severe clinical manifestations, mortality, and sequelae. In this review, we primarily elaborate on the pathophysiological mechanisms of thrombosis in COVID-19 patients with cancer, emphasize the role of microparticles (MPs) and phosphatidylserine (PS) in coagulation, and propose an antithrombotic strategy. The coagulation mechanisms of COVID-19 and cancer synergistically amplify the coagulation cascade, and collectively promotes pulmonary microvascular occlusion. During systemic coagulation, the virus activates immune cells to release abundant proinflammatory cytokines, referred to as cytokine storm, resulting in the apoptosis of tumor and blood cells and subsequent MPs release. Additionally, we highlight that tumor cells contribute to MPs and coagulation by apoptosis owing to insufficient blood supply. A positive feedback loop of cytokines storm and MPs storm promotes microvascular coagulation storm, leading to microthrombi formation and inadequate blood perfusion. Microthrombi-damaged endothelial cells (ECs), tumor, and blood cells further aggravate the apoptosis of the cells and facilitate MPs storm. PS, especially on MPs, plays a pivotal role in the blood coagulation process, contributing to clot initiation, amplification, and propagation. Since coagulation is a common pathway of COVID-19 and cancer, and associated with mortality, patients would benefit from antithrombotic therapy. The above results lead us to assert that early stage antithrombotic therapy is optimal. This strategy is likely to maintain blood flow patency contributing to viral clearance, attenuating the formation of cytokines and MPs storm, maintaining oxygen saturation, and avoiding the progress of the disease.
Keywords: COVID-19; cancer; microparticles; phosphatidylserine; thrombosis; treatment strategy.
Copyright © 2022 Jing, Zuo, Novakovic and Shi.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
The cross-talk of lung and heart complications in COVID-19: Endothelial cells dysfunction, thrombosis, and treatment.Front Cardiovasc Med. 2022 Aug 5;9:957006. doi: 10.3389/fcvm.2022.957006. eCollection 2022. Front Cardiovasc Med. 2022. PMID: 35990983 Free PMC article. Review.
-
Circulating Microparticles in the Pathogenesis and Early Anticoagulation of Thrombosis in COVID-19 With Kidney Injury.Front Cell Dev Biol. 2022 Jan 18;9:784505. doi: 10.3389/fcell.2021.784505. eCollection 2021. Front Cell Dev Biol. 2022. PMID: 35118071 Free PMC article. Review.
-
Pathophysiological mechanisms of thrombosis in acute and long COVID-19.Front Immunol. 2022 Nov 16;13:992384. doi: 10.3389/fimmu.2022.992384. eCollection 2022. Front Immunol. 2022. PMID: 36466841 Free PMC article. Review.
-
Neutrophil extracellular traps (NETs): the role of inflammation and coagulation in COVID-19.Am J Transl Res. 2021 Aug 15;13(8):8575-8588. eCollection 2021. Am J Transl Res. 2021. PMID: 34539980 Free PMC article. Review.
-
Long COVID: The Nature of Thrombotic Sequelae Determines the Necessity of Early Anticoagulation.Front Cell Infect Microbiol. 2022 Apr 5;12:861703. doi: 10.3389/fcimb.2022.861703. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 35449732 Free PMC article. Review.
Cited by
-
Elevated risk of thrombotic manifestations of SARS-CoV-2 infection in cancer patients: A literature review.EXCLI J. 2022 Jun 30;21:906-920. doi: 10.17179/excli2022-5073. eCollection 2022. EXCLI J. 2022. PMID: 36172074 Free PMC article. Review.
-
The functional role of soluble proteins acquired by extracellular vesicles.J Extracell Biol. 2022 Mar 16;1(1):e34. doi: 10.1002/jex2.34. eCollection 2022 Jan. J Extracell Biol. 2022. PMID: 38938684 Free PMC article. Review.
-
Extracellular Vesicles: The Invisible Heroes and Villains of COVID-19 Central Neuropathology.Adv Sci (Weinh). 2024 Mar;11(10):e2305554. doi: 10.1002/advs.202305554. Epub 2023 Dec 24. Adv Sci (Weinh). 2024. PMID: 38143270 Free PMC article. Review.
-
The Endothelium and COVID-19: An Increasingly Clear Link Brief Title: Endotheliopathy in COVID-19.Int J Mol Sci. 2022 May 31;23(11):6196. doi: 10.3390/ijms23116196. Int J Mol Sci. 2022. PMID: 35682871 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

