STK35 Gene Therapy Attenuates Endothelial Dysfunction and Improves Cardiac Function in Diabetes
- PMID: 35097018
- PMCID: PMC8792894
- DOI: 10.3389/fcvm.2021.798091
STK35 Gene Therapy Attenuates Endothelial Dysfunction and Improves Cardiac Function in Diabetes
Retraction in
-
Retraction: STK35 gene therapy attenuates endothelial dysfunction and improves cardiac function in diabetes.Front Cardiovasc Med. 2023 May 9;10:1208579. doi: 10.3389/fcvm.2023.1208579. eCollection 2023. Front Cardiovasc Med. 2023. PMID: 37229227 Free PMC article.
Abstract
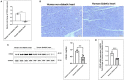
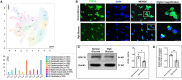
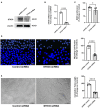
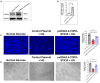
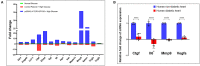
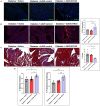
Diabetic cardiomyopathy (DCM) is characterized by microvascular pathology and interstitial fibrosis that leads to progressive heart failure. The mechanisms underlying DCM pathogenesis remain obscure, and no effective treatments for the disease have been available. In the present study, we observed that STK35, a novel kinase, is decreased in the diabetic human heart. High glucose treatment, mimicking hyperglycemia in diabetes, downregulated STK35 expression in mouse cardiac endothelial cells (MCEC). Knockdown of STK35 attenuated MCEC proliferation, migration, and tube formation, whereas STK35 overexpression restored the high glucose-suppressed MCEC migration and tube formation. Angiogenesis gene PCR array analysis revealed that HG downregulated the expression of several angiogenic genes, and this suppression was fully restored by STK35 overexpression. Intravenous injection of AAV9-STK35 viral particles successfully overexpressed STK35 in diabetic mouse hearts, leading to increased vascular density, suppression of fibrosis in the heart, and amelioration of left ventricular function. Altogether, our results suggest that hyperglycemia downregulates endothelial STK35 expression, leading to microvascular dysfunction in diabetic hearts, representing a novel mechanism underlying DCM pathogenesis. Our study also emerges STK35 is a novel gene therapeutic target for preventing and treating DCM.
Keywords: STK35; angiogenesis; cardiac function; diabetes; gene therapy; serine threonine kinase.
Copyright © 2022 Joladarashi, Zhu, Willman, Nash, Cimini, Thandavarayan, Youker, Song, Ren, Li, Kishore, Krishnamurthy and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Islam Z, Akter S, Inoue Y, Hu H, Kuwahara K, Nakagawa T, et al. Prediabetes, diabetes, and the risk of all-cause and cause-specific mortality in a Japanese working population: Japan epidemiology collaboration on occupational health study. Diabetes Care. (2021) 44:757–64. 10.2337/dc20-1213 - DOI - PMC - PubMed
-
- Morimoto A, Onda Y, Nishimura R, Sano H, Utsunomiya K, Tajima N, et al. Cause-specific mortality trends in a nationwide population-based cohort of childhood-onset type 1 diabetes in Japan during 35 years of follow-up: the DERI Mortality Study. Diabetologia. (2013) 56:2171–5. 10.1007/s00125-013-3001-2 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

