Facile isolation of high-affinity nanobodies from synthetic libraries using CDR-swapping mutagenesis
- PMID: 35098159
- PMCID: PMC8783142
- DOI: 10.1016/j.xpro.2021.101101
Facile isolation of high-affinity nanobodies from synthetic libraries using CDR-swapping mutagenesis
Abstract
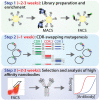
The generation of high-affinity nanobodies for diverse biomedical applications typically requires immunization or affinity maturation. Here, we report a simple protocol using complementarity-determining region (CDR)-swapping mutagenesis to isolate high-affinity nanobodies from common framework libraries. This approach involves shuffling the CDRs of low-affinity variants during the sorting of yeast-displayed libraries to directly isolate high-affinity nanobodies without the need for lead isolation and optimization. We expect this approach, which we demonstrate for SARS-CoV-2 neutralizing nanobodies, will simplify the generation of high-affinity nanobodies. For complete details on the use and execution of this profile, please refer to Zupancic et al. (2021).
Keywords: Antibody; Biotechnology and bioengineering; Cell Biology; Flow Cytometry/Mass Cytometry; Molecular Biology; Protein Biochemistry; Protein expression and purification.
© 2021 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



Comment on
-
Directed evolution of potent neutralizing nanobodies against SARS-CoV-2 using CDR-swapping mutagenesis.Cell Chem Biol. 2021 Sep 16;28(9):1379-1388.e7. doi: 10.1016/j.chembiol.2021.05.019. Epub 2021 Jun 24. Cell Chem Biol. 2021. PMID: 34171229 Free PMC article.
References
-
- Benatuil L., Perez J.M., Belk J., Hsieh C.-M. An improved yeast transformation method for the generation of very large human antibody libraries. Protein Eng. Des. Sel. 2010;23:155–159. - PubMed
-
- L’Abbé D., Bisson L., Gervais C., Grazzini E., Durocher Y. Transient gene expression in suspension HEK293-EBNA1 Cells. Methods Mol. Biol. 2018;1850:1–16. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

