Gene Therapy Potential for Genetic Disorders of Surfactant Dysfunction
- PMID: 35098209
- PMCID: PMC8798122
- DOI: 10.3389/fgeed.2021.785829
Gene Therapy Potential for Genetic Disorders of Surfactant Dysfunction
Abstract
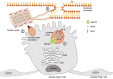
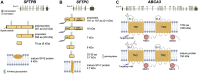
Pulmonary surfactant is critically important to prevent atelectasis by lowering the surface tension of the alveolar lining liquid. While respiratory distress syndrome (RDS) is common in premature infants, severe RDS in term and late preterm infants suggests an underlying genetic etiology. Pathogenic variants in the genes encoding key components of pulmonary surfactant including surfactant protein B (SP-B, SFTPB gene), surfactant protein C (SP-C, SFTPC gene), and the ATP-Binding Cassette transporter A3 (ABCA3, ABCA3 gene) result in severe neonatal RDS or childhood interstitial lung disease (chILD). These proteins play essential roles in pulmonary surfactant biogenesis and are expressed in alveolar epithelial type II cells (AEC2), the progenitor cell of the alveolar epithelium. SP-B deficiency most commonly presents in the neonatal period with severe RDS and requires lung transplantation for survival. SFTPC mutations act in an autosomal dominant fashion and more commonly presents with chILD or idiopathic pulmonary fibrosis than neonatal RDS. ABCA3 deficiency often presents as neonatal RDS or chILD. Gene therapy is a promising option to treat monogenic lung diseases. Successes and challenges in developing gene therapies for genetic disorders of surfactant dysfunction include viral vector design and tropism for target cell types. In this review, we explore adeno-associated virus (AAV), lentiviral, and adenoviral (Ad)-based vectors as delivery vehicles. Both gene addition and gene editing strategies are compared to best design treatments for lung diseases resulting from pathogenic variants in the SFTPB, SFTPC, and ABCA3 genes.
Keywords: AEC2; AT2; ATII; alveoli; pulmonary disease; surfactant deficiency; viral vectors.
Copyright © 2022 Cooney, Wambach, Sinn and McCray.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. PM is on the SAB consults and performs sponsored research for Spirovant Sciences.
Figures
References
-
- Barnett R. C., Lin X., Barravecchia M., Norman R. A., de Mesy Bentley K. L., Fazal F., et al. (2017). Featured Article: Electroporation-Mediated Gene Delivery of Surfactant Protein B (Sp-b) Restores Expression and Improves Survival in Mouse Model of Sp-B Deficiency. Exp. Biol. Med. (Maywood) 242, 1345–1354. 10.1177/1535370217713000 - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

