Acetylcysteine has No Mechanistic Effect in Patients at Risk of Contrast-Induced Nephropathy: A Failure of Academic Clinical Science
- PMID: 35098531
- PMCID: PMC9306485
- DOI: 10.1002/cpt.2541
Acetylcysteine has No Mechanistic Effect in Patients at Risk of Contrast-Induced Nephropathy: A Failure of Academic Clinical Science
Abstract
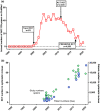
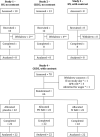
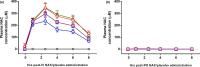
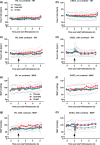
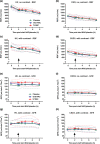
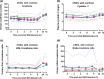
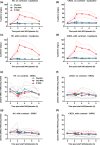
Contrast-induced nephropathy (CIN) is a major complication of imaging in patients with chronic kidney disease (CKD). The publication of an academic randomized controlled trial (RCT; n = 83) reporting oral (N)-acetylcysteine (NAC) to reduce CIN led to > 70 clinical trials, 23 systematic reviews, and 2 large RCTs showing no benefit. However, no mechanistic studies were conducted to determine how NAC might work; proposed mechanisms included renal artery vasodilatation and antioxidant boosting. We evaluated the proposed mechanisms of NAC action in participants with healthy and diseased kidneys. Four substudies were performed. Two randomized, double-blind, placebo-controlled, three-period crossover studies (n = 8) assessed the effect of oral and intravenous (i.v.) NAC in healthy kidneys in the presence/absence of iso-osmolar contrast (iodixanol). A third crossover study in patients with CKD stage III (CKD3) (n = 8) assessed the effect of oral and i.v. NAC without contrast. A three-arm randomized, double-blind, placebo-controlled parallel-group study, recruiting patients with CKD3 (n = 66) undergoing coronary angiography, assessed the effect of oral and i.v. NAC in the presence of contrast. We recorded systemic (blood pressure and heart rate) and renal (renal blood flow (RBF) and glomerular filtration rate (GFR)) hemodynamics, and antioxidant status, plus biomarkers of renal injury in patients with CKD3 undergoing angiography. Primary outcome for all studies was RBF over 8 hours after the start of i.v. NAC/placebo. NAC at doses used in previous trials of renal prophylaxis was essentially undetectable in plasma after oral administration. In healthy volunteers, i.v. NAC, but not oral NAC, increased blood pressure (mean area under the curve (AUC) mean arterial pressure (MAP): mean difference 29 h⋅mmHg, P = 0.019 vs. placebo), heart rate (28 h⋅bpm, P < 0.001), and RBF (714 h⋅mL/min, 8.0% increase, P = 0.006). Renal vasodilatation also occurred in the presence of contrast (RBF 917 h⋅mL/min, 12% increase, P = 0.005). In patients with CKD3 without contrast, only a rise in heart rate (34 h⋅bpm, P = 0.010) and RBF (288 h⋅mL/min, 6.0% increase, P = 0.001) occurred with i.v. NAC, with no significant effect on blood pressure (MAP rise 26 h⋅mmHg, P = 0.156). Oral NAC showed no effect. In patients with CKD3 receiving contrast, i.v. NAC increased blood pressure (MAP rise 52 h⋅mmHg, P = 0.008) but had no effect on RBF (151 h⋅mL/min, 3.0% increase, P = 0.470), GFR (29 h⋅mL/min/1.73m², P = 0.122), or markers of renal injury. Neither i.v. nor oral NAC affected plasma antioxidant status. We found oral NAC to be poorly absorbed and have no reno-protective effects. Intravenous, not oral, NAC caused renal artery vasodilatation in healthy volunteers but offered no protection to patients with CKD3 at risk of CIN. These findings emphasize the importance of mechanistic clinical studies before progressing to RCTs for novel interventions. Thousands were recruited to academic clinical trials without the necessary mechanistic studies being performed to confirm the approach had any chance of working.
© 2022 The Authors. Clinical Pharmacology & Therapeutics published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
The authors declared no competing interests for this work.
Figures
References
-
- Parfrey, P. The clinical epidemiology of contrast‐induced nephropathy. Cardiovasc. Intervent. Radiol. 28(Suppl 2), S3–S11 (2005). - PubMed
-
- Weisbord, S.D. et al. Associations of increases in serum creatinine with mortality and length of hospital stay after coronary angiography. J. Am. Soc. Nephrol. 17, 2871–2877 (2006). - PubMed
-
- Brown, J.R. , Rezaee, M.E. , Nichols, E.L. , Marshall, E.J. , Siew, E.D. & Matheny, M.E. Incidence and in‐hospital mortality of acute kidney injury (AKI) and dialysis‐requiring AKI (AKI‐D) after cardiac catheterization in the National Inpatient Sample. J. Am. Heart Assoc. 5, e002739 (2016). - PMC - PubMed
-
- Gorelik, Y. , Bloch‐Isenberg, N. , Yaseen, H. , Heyman, S.N. & Khamaisi, M. Acute kidney injury after radiocontrast‐enhanced computerized tomography in hospitalized patients with advanced renal failure: a propensity‐score‐matching analysis. Invest. Radiol. 55, 677–687 (2020). - PubMed
-
- Solomon, R. Acute kidney injury: antioxidants do not PRESERVE kidney function after contrast exposure. Nat. Rev. Nephrol. 14, 148–149 (2018). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

