Muscle systems and motility of early animals highlighted by cnidarians from the basal Cambrian
- PMID: 35098925
- PMCID: PMC8837203
- DOI: 10.7554/eLife.74716
Muscle systems and motility of early animals highlighted by cnidarians from the basal Cambrian
Abstract
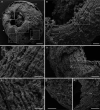
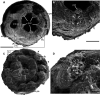
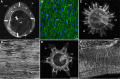
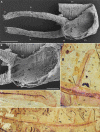
Although fossil evidence suggests that various animal groups were able to move actively through their environment in the early stages of their evolution, virtually no direct information is available on the nature of their muscle systems. The origin of jellyfish swimming, for example, is of great interest to biologists. Exceptionally preserved muscles are described here in benthic peridermal olivooid medusozoans from the basal Cambrian of China (Kuanchuanpu Formation, ca. 535 Ma) that have direct equivalent in modern medusozoans. They consist of circular fibers distributed over the bell surface (subumbrella) and most probably have a myoepithelial origin. This is the oldest record of a muscle system in cnidarians and more generally in animals. This basic system was probably co-opted by early Cambrian jellyfish to develop capacities for jet-propelled swimming within the water column. Additional lines of fossil evidence obtained from ecdysozoans (worms and panarthropods) show that the muscle systems of early animals underwent a rapid diversification through the early Cambrian and increased their capacity to colonize a wide range of habitats both within the water column and sediment at a critical time of their evolutionary radiation.
Keywords: basal Cambrian; evolutionary biology; jellyfish swimming; motility; muscle system; small shelly fossils.
© 2022, Wang et al.
Conflict of interest statement
XW, JV, XY, LL, QO, XS, TK, JH No competing interests declared
Figures








References
-
- Aria C, Zhao F, Zhu M. Fuxianhuiids are mandibulates and share affinities with total-group Myriapoda. Journal of the Geological Society. 2021;178:jgs2020–246. doi: 10.1144/jgs2020-246. - DOI
-
- Bengtson S, Yue Z. Fossilized metazoan embryos from the earliest Cambrian. Science. 1997;277:1645–1648. doi: 10.1126/science.277.5332.1645. - DOI
-
- Brusca RC, Moore W, Shuster SM. Invertebrates. Sinauer AssociatesInc; 2016.
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources

