Targeting transforming growth factor-β2 by antisense oligodeoxynucleotide accelerates T cell-mediated tumor rejection in a humanized mouse model of triple-negative breast cancer
- PMID: 35099588
- PMCID: PMC10992746
- DOI: 10.1007/s00262-022-03157-w
Targeting transforming growth factor-β2 by antisense oligodeoxynucleotide accelerates T cell-mediated tumor rejection in a humanized mouse model of triple-negative breast cancer
Abstract
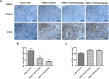
Transforming growth factor-beta (TGF-β) pathway mediates suppression of antitumor immunity and is associated with poor prognosis in triple-negative breast cancer (TNBC). In this study, we generated a humanized animal model by transplanting human peripheral blood mononuclear cells into immunodeficient mice followed by inoculation of MDA-MB-231 cells and subsequently analyzed the role of TGF-β2 in the interaction between human T cells and human tumor cells. Following reconstitution of the human immune system, inhibition of TGF-β signaling by TGF-β2 antisense oligodeoxynucleotide (TASO) resulted in accelerated tumor growth inhibition. TGF-β2 inhibition also resulted in downregulation of peripheral Foxp3 + regulatory T cells (Treg), whereas no effect was seen in the expression of CD8 + cytotoxic T cells. Analysis of the TASO-treated mice serum revealed elevated levels of human IFN-γ and reduced levels of human IL-10 and TGF-β2. Moreover, TGF-β2 inhibition resulted in increased CD8 + T cell infiltration, whereas the reduced infiltration of Tregs into the tumor partly resulted from decreased expression of CCL22. Decreased intratumoral Tregs facilitated the activation of cytotoxic T cells, associated with increased granzyme B expression. These results indicate that TASO potentiated T cell-mediated antitumor immunity, and it is proposed that TGF-β2 may be a promising target in the immunotherapeutic strategy of TNBC.
Keywords: Antitumor immunity; Breast cancer; Humanized model; TGF-β2 antisense.
© 2022. The Author(s), under exclusive licence to Springer-Verlag GmbH Germany, part of Springer Nature.
Conflict of interest statement
Jun-Eui Park, Jihye Koo, and Tae Hun Kim are current employees of Autotelic Bio, Inc. All other authors do not have any conflicts of interest to declare.
Figures






References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

