Nanozyme-Based Enhanced Cancer Immunotherapy
- PMID: 35099759
- PMCID: PMC8971237
- DOI: 10.1007/s13770-022-00430-y
Nanozyme-Based Enhanced Cancer Immunotherapy
Abstract
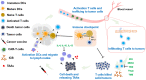
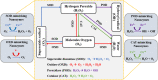
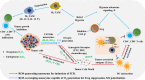
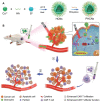
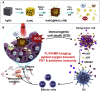
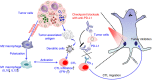
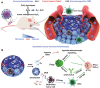
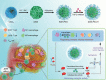
Catalytic nanoparticles with natural enzyme-mimicking properties, known as nanozymes, have emerged as excellent candidate materials for cancer immunotherapy. Owing to their enzymatic activities, artificial nanozymes not only serve as responsive carriers to load drugs and therapeutic molecules for cancer treatment, but also act as enzymes for modulating the immunosuppression of the tumor microenvironment (TME) via the catalytic activities of catalase, peroxidase, superoxide dismutase, and oxidase. The immunosuppressive pro-tumor TME can be reversed to the immunoactive anti-tumor TME by utilizing both reactive oxygen species (ROS)-generating and ROS-scavenging nanozymes, which enhance the efficacy of cancer immunotherapy. In this review, we introduce representative ROS-generating and ROS-scavenging nanozymes and discuss how artificial nanozymes respond to the conditions of the TME. Based on the mutual interaction between nanozymes and TME, recent therapeutic pathways to provoke anti-cancer immune responses using nanozymes are discussed.
Keywords: Cancer immunotherapy; Immunogenic cell death; Nanozymes; Reactive oxygen species; Tumor microenvironment.
© 2022. The Korean Tissue Engineering and Regenerative Medicine Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Sahin U, Türeci Ö. Personalized vaccines for cancer immunotherapy. Science. 2018;359:1355–1360. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
