Cost-Effectiveness of Long-Acting Injectable HIV Preexposure Prophylaxis in the United States : A Cost-Effectiveness Analysis
- PMID: 35099992
- PMCID: PMC9087297
- DOI: 10.7326/M21-1548
Cost-Effectiveness of Long-Acting Injectable HIV Preexposure Prophylaxis in the United States : A Cost-Effectiveness Analysis
Abstract
Background: The HIV Prevention Trials Network (HPTN) 083 trial demonstrated the superiority of long-acting injectable cabotegravir (CAB-LA) compared with oral emtricitabine-tenofovir disoproxil fumarate (F/TDF) for HIV preexposure prophylaxis (PrEP).
Objective: To identify the maximum price premium (that is, greatest possible price differential) that society should be willing to accept for the additional benefits of CAB-LA over tenofovir-based PrEP among men who have sex with men and transgender women (MSM/TGW) in the United States.
Design: Simulation, cost-effectiveness analysis.
Data sources: Trial and published data, including estimated HIV incidence (5.32, 1.33, and 0.26 per 100 person-years for off PrEP, generic F/TDF and branded emtricitabine-tenofovir alafenamide (F/TAF), and CAB-LA, respectively); 28% 6-year PrEP retention. Annual base-case drug costs: $360 and $16 800 for generic F/TDF and branded F/TAF. Fewer side effects with branded F/TAF versus generic F/TDF were assumed.
Target population: 476 700 MSM/TGW at very high risk for HIV (VHR).
Time horizon: 10 years.
Perspective: Health care system.
Intervention: CAB-LA versus generic F/TDF or branded F/TAF for HIV PrEP.
Outcome measures: Primary transmissions, quality-adjusted life-years (QALYs), costs (2020 U.S. dollars), incremental cost-effectiveness ratios (ICERs; U.S. dollars per QALY), maximum price premium for CAB-LA versus tenofovir-based PrEP.
Results of base-case analysis: Compared with generic F/TDF (or branded F/TAF), CAB-LA increased life expectancy by 28 000 QALYs (26 000 QALYs) among those at VHR. Branded F/TAF cost more per QALY gained than generic F/TDF compared with no PrEP. At 10 years, CAB-LA could achieve an ICER of at most $100 000 per QALY compared with generic F/TDF at a maximum price premium of $3700 per year over generic F/TDF (CAB-LA price <$4100 per year).
Results of sensitivity analysis: In a PrEP-eligible population at high risk for HIV, rather than at VHR (n = 1 906 800; off PrEP incidence: 1.54 per 100 person-years), CAB-LA could achieve an ICER of at most $100 000 per QALY versus generic F/TDF at a maximum price premium of $1100 per year over generic F/TDF (CAB-LA price <$1500 per year).
Limitation: Uncertain clinical and economic benefits of averting future transmissions.
Conclusion: Effective oral PrEP limits the additional price society should be willing to pay for CAB-LA.
Primary funding source: FHI 360; Eunice Kennedy Shriver National Institute of Child Health and Human Development; National Institute of Allergy and Infectious Diseases; National Heart, Lung, and Blood Institute; National Institute on Drug Abuse; the Reich HIV Scholar Award; and the Steve and Deborah Gorlin MGH Research Scholars Award.
Figures
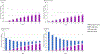
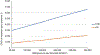

Comment in
-
Determining the Most Appropriate Use of Available Regimens for HIV Preexposure Prophylaxis.Ann Intern Med. 2022 Apr;175(4):600-601. doi: 10.7326/M22-0057. Epub 2022 Feb 1. Ann Intern Med. 2022. PMID: 35099987 No abstract available.
Similar articles
-
Cost-effective pricing of long-acting injectable HIV pre-exposure prophylaxis for adolescent girls and young women in South Africa: a model-based analysis.Lancet Glob Health. 2025 Jul;13(7):e1230-e1239. doi: 10.1016/S2214-109X(25)00119-6. Epub 2025 May 26. Lancet Glob Health. 2025. PMID: 40441175 Free PMC article.
-
Comparative Pricing of Branded Tenofovir Alafenamide-Emtricitabine Relative to Generic Tenofovir Disoproxil Fumarate-Emtricitabine for HIV Preexposure Prophylaxis: A Cost-Effectiveness Analysis.Ann Intern Med. 2020 May 5;172(9):583-590. doi: 10.7326/M19-3478. Epub 2020 Mar 10. Ann Intern Med. 2020. PMID: 32150602 Free PMC article.
-
Cost-effectiveness of cabotegravir versus tenofovir alafenamide plus emtricitabine versus tenofovir disoproxil fumarate plus emtricitabine for pre-exposure prophylaxis to prevent HIV-1 transmission in gay, bisexual and other men that have sex with men.Enferm Infecc Microbiol Clin (Engl Ed). 2025 Aug-Sep;43(7):416-425. doi: 10.1016/j.eimce.2024.12.014. Epub 2025 May 22. Enferm Infecc Microbiol Clin (Engl Ed). 2025. PMID: 40410032
-
The effectiveness and cost-effectiveness of carmustine implants and temozolomide for the treatment of newly diagnosed high-grade glioma: a systematic review and economic evaluation.Health Technol Assess. 2007 Nov;11(45):iii-iv, ix-221. doi: 10.3310/hta11450. Health Technol Assess. 2007. PMID: 17999840
-
Adherence and HIV Protection Thresholds for Emtricitabine and Tenofovir Disoproxil Fumarate Preexposure Prophylaxis among Cisgender Women: A Systematic Review.Curr HIV/AIDS Rep. 2024 Oct;21(5):264-281. doi: 10.1007/s11904-024-00705-0. Epub 2024 Aug 9. Curr HIV/AIDS Rep. 2024. PMID: 39120667
Cited by
-
Development and Validation of a Simulation Model for Treatment to Maintain Remission in Antineutrophil Cytoplasmic Antibody-Associated Vasculitis.Arthritis Care Res (Hoboken). 2023 Sep;75(9):1976-1985. doi: 10.1002/acr.25088. Epub 2023 Feb 24. Arthritis Care Res (Hoboken). 2023. PMID: 36645017 Free PMC article.
-
Responding to the HIV Health Literacy Needs of Clients in Substance Use Treatment: The Role of Universal PrEP Education in HIV Health and Prevention.Int J Environ Res Public Health. 2023 Oct 7;20(19):6893. doi: 10.3390/ijerph20196893. Int J Environ Res Public Health. 2023. PMID: 37835163 Free PMC article.
-
Perspectives on long-acting formulations of pre-exposure prophylaxis (PrEP) among men who have sex with men who are non-adherent to daily oral PrEP in the United States.BMC Public Health. 2023 Aug 28;23(1):1643. doi: 10.1186/s12889-023-16382-4. BMC Public Health. 2023. PMID: 37641018 Free PMC article.
-
Population-level impact of expanding PrEP coverage by offering long-acting injectable PrEP to MSM in three high-resource settings: a model comparison analysis.J Int AIDS Soc. 2023 Jul;26 Suppl 2(Suppl 2):e26109. doi: 10.1002/jia2.26109. J Int AIDS Soc. 2023. PMID: 37439080 Free PMC article.
-
Readiness to Provide Oral and Injectable PrEP for Sexual and Gender Minority Youth Among Healthcare Providers and Clinics in the U.S. Northeast.J Adolesc Health. 2023 May;72(5):722-729. doi: 10.1016/j.jadohealth.2022.11.246. Epub 2023 Jan 4. J Adolesc Health. 2023. PMID: 36604205 Free PMC article.
References
-
- Horn T Drug pricing in the age of generics [Internet]. ACTHIV: The American Conference for the Treatment of HIV; 2019. Apr 13 [cited 2021 Mar 3]; Miami, Florida. Available from: http://www.acthiv.org/wp-content/uploads/2019/04/Tim_Horn_09_30.pdf
-
- Hoover KW. Trends in Truvada and Descovy prescriptions for PrEP in the United States, 2014–2020 [Internet]. Conference on Retroviruses and Opportunistic Infections; 2021. Mar 6 [cited 2021 Oct 21]; Virtual. Available from: https://www.croiconference.org/abstract/trends-in-truvada-and-descovy-pr...
-
- U.S. House of Representatives Committee Repository. Hearing: HIV prevention drug: billions in corporate profits after millions in taxpayer investments [Internet]. 2019. [cited 2021 Mar 3]. Available from: https://docs.house.gov/Committee/Calendar/ByEvent.aspx?EventID=109486
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
