Analytical kinetic model of native tandem promoters in E. coli
- PMID: 35100257
- PMCID: PMC8830795
- DOI: 10.1371/journal.pcbi.1009824
Analytical kinetic model of native tandem promoters in E. coli
Abstract
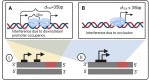
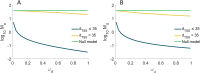
Closely spaced promoters in tandem formation are abundant in bacteria. We investigated the evolutionary conservation, biological functions, and the RNA and single-cell protein expression of genes regulated by tandem promoters in E. coli. We also studied the sequence (distance between transcription start sites 'dTSS', pause sequences, and distances from oriC) and potential influence of the input transcription factors of these promoters. From this, we propose an analytical model of gene expression based on measured expression dynamics, where RNAP-promoter occupancy times and dTSS are the key regulators of transcription interference due to TSS occlusion by RNAP at one of the promoters (when dTSS ≤ 35 bp) and RNAP occupancy of the downstream promoter (when dTSS > 35 bp). Occlusion and downstream promoter occupancy are modeled as linear functions of occupancy time, while the influence of dTSS is implemented by a continuous step function, fit to in vivo data on mean single-cell protein numbers of 30 natural genes controlled by tandem promoters. The best-fitting step is at 35 bp, matching the length of DNA occupied by RNAP in the open complex formation. This model accurately predicts the squared coefficient of variation and skewness of the natural single-cell protein numbers as a function of dTSS. Additional predictions suggest that promoters in tandem formation can cover a wide range of transcription dynamics within realistic intervals of parameter values. By accurately capturing the dynamics of these promoters, this model can be helpful to predict the dynamics of new promoters and contribute to the expansion of the repertoire of expression dynamics available to synthetic genetic constructs.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures







References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

