Redundant neural circuits regulate olfactory integration
- PMID: 35100258
- PMCID: PMC8830790
- DOI: 10.1371/journal.pgen.1010029
Redundant neural circuits regulate olfactory integration
Abstract
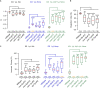
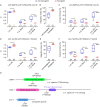
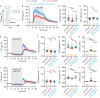
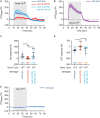
Olfactory integration is important for survival in a natural habitat. However, how the nervous system processes signals of two odorants present simultaneously to generate a coherent behavioral response is poorly understood. Here, we characterize circuit basis for a form of olfactory integration in Caenorhabditis elegans. We find that the presence of a repulsive odorant, 2-nonanone, that signals threat strongly blocks the attraction of other odorants, such as isoamyl alcohol (IAA) or benzaldehyde, that signal food. Using a forward genetic screen, we found that genes known to regulate the structure and function of sensory neurons, osm-5 and osm-1, played a critical role in the integration process. Loss of these genes mildly reduces the response to the repellent 2-nonanone and disrupts the integration effect. Restoring the function of OSM-5 in either AWB or ASH, two sensory neurons known to mediate 2-nonanone-evoked avoidance, is sufficient to rescue. Sensory neurons AWB and downstream interneurons AVA, AIB, RIM that play critical roles in olfactory sensorimotor response are able to process signals generated by 2-nonanone or IAA or the mixture of the two odorants and contribute to the integration. Thus, our results identify redundant neural circuits that regulate the robust effect of a repulsive odorant to block responses to attractive odorants and uncover the neuronal and cellular basis for this complex olfactory task.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

