A robust biostatistical method leverages informative but uncertainly determined qPCR data for biomarker detection, early diagnosis, and treatment
- PMID: 35100319
- PMCID: PMC8803186
- DOI: 10.1371/journal.pone.0263070
A robust biostatistical method leverages informative but uncertainly determined qPCR data for biomarker detection, early diagnosis, and treatment
Abstract
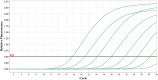
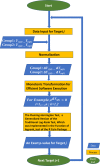
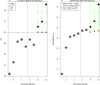
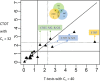
As a common medium-throughput technique, qPCR (quantitative real-time polymerase chain reaction) is widely used to measure levels of nucleic acids. In addition to accurate and complete data, experimenters have unavoidably observed some incomplete and uncertainly determined qPCR data because of intrinsically low overall amounts of biological materials, such as nucleic acids present in biofluids. When there are samples with uncertainly determined qPCR data, some investigators apply the statistical complete-case method by excluding the subset of samples with uncertainly determined data from analysis (CO), while others simply choose not to analyze (CNA) these datasets altogether. To include as many observations as possible in analysis for interesting differential changes between groups, some investigators set incomplete observations equal to the maximum quality qPCR cycle (MC), such as 32 and 40. Although straightforward, these methods may decrease the sample size, skew the data distribution, and compromise statistical power and research reproducibility across replicate qPCR studies. To overcome the shortcomings of the existing, commonly-used qPCR data analysis methods and to join the efforts in advancing statistical analysis in rigorous preclinical research, we propose a robust nonparametric statistical cycle-to-threshold method (CTOT) to analyze incomplete qPCR data for two-group comparisons. CTOT incorporates important characteristics of qPCR data and time-to-event statistical methodology, resulting in a novel analytical method for qPCR data that is built around good quality data from all subjects, certainly determined or not. Considering the benchmark full data (BFD), we compared the abilities of CTOT, CO, MC, and CNA statistical methods to detect interesting differential changes between groups with informative but uncertainly determined qPCR data. Our simulations and applications show that CTOT improves the power of detecting and confirming differential changes in many situations over the three commonly used methods without excess type I errors. The robust nonparametric statistical method of CTOT helps leverage qPCR technology and increase the power to detect differential changes that may assist decision making with respect to biomarker detection and early diagnosis, with the goal of improving the management of patient healthcare.
Conflict of interest statement
This work was conducted with the internal funding, NCTR protocol E0772101, of the U.S. Food and Drug Administration, a U.S. government agency. The authors have declared that no competing interests exist. The views presented in this article do not necessarily reflect those of the U.S. Food and Drug Administration. Any mention of commercial products is for clarification and is not intended as an endorsement. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures




Similar articles
-
Reproducibility challenges for biomarker detection with uncertain but informative experimental data.Biomark Med. 2020 Sep;14(13):1255-1263. doi: 10.2217/bmm-2019-0599. Biomark Med. 2020. PMID: 33021389
-
Evaluation of qPCR curve analysis methods for reliable biomarker discovery: bias, resolution, precision, and implications.Methods. 2013 Jan;59(1):32-46. doi: 10.1016/j.ymeth.2012.08.011. Epub 2012 Sep 3. Methods. 2013. PMID: 22975077
-
Subgroup analyses in randomised controlled trials: quantifying the risks of false-positives and false-negatives.Health Technol Assess. 2001;5(33):1-56. doi: 10.3310/hta5330. Health Technol Assess. 2001. PMID: 11701102 Review.
-
Why the need for qPCR publication guidelines?--The case for MIQE.Methods. 2010 Apr;50(4):217-26. doi: 10.1016/j.ymeth.2009.12.006. Epub 2009 Dec 16. Methods. 2010. PMID: 20025972
-
[Aiming for zero blindness].Nippon Ganka Gakkai Zasshi. 2015 Mar;119(3):168-93; discussion 194. Nippon Ganka Gakkai Zasshi. 2015. PMID: 25854109 Review. Japanese.
Cited by
-
The MCTOT app: A publicly available tool for statistical cycle-to-threshold analysis and inference of informative but uncertainly determined qPCR data.PLoS One. 2025 Sep 2;20(9):e0330729. doi: 10.1371/journal.pone.0330729. eCollection 2025. PLoS One. 2025. PMID: 40892763 Free PMC article.
References
-
- Silva CS, Chang CW, Williams D, Porter-Gill P, da Costa GG, Camacho L. Effects of a 28-day dietary co-exposure to melamine and cyanuric acid on the levels of serum microRNAs in male and female Fisher 344 rats. Food Chem Toxicol. 2016;98:11–6. doi: 10.1016/j.fct.2016.09.013 PubMed PMID: WOS:000388054000003. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

