Deleterious effect of bone marrow-resident macrophages on hematopoietic stem cells in response to total body irradiation
- PMID: 35100346
- PMCID: PMC8941479
- DOI: 10.1182/bloodadvances.2021005983
Deleterious effect of bone marrow-resident macrophages on hematopoietic stem cells in response to total body irradiation
Abstract
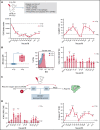
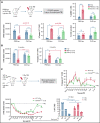
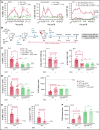
Bone marrow (BM) resident macrophages interact with a population of long-term hematopoietic stem cells (LT-HSCs) but their role on LT-HSC properties after stress is not well defined. Here, we show that a 2 Gy-total body irradiation (TBI)-mediated death of LT-HSCs is associated with increased percentages of LT-HSCs with reactive oxygen species (ROS) and of BM resident macrophages producing nitric oxide (NO), resulting in an increased percentage of LT-HSCs with endogenous cytotoxic peroxynitrites. Pharmacological or genetic depletion of BM resident macrophages impairs the radio-induced increases in the percentage of both ROS+ LT-HSCs and peroxynitrite+ LT-HSCs and results in a complete recovery of a functional pool of LT-HSCs. Finally, we show that after a 2 Gy-TBI, a specific decrease of NO production by BM resident macrophages improves the LT-HSC recovery, whereas an exogenous NO delivery decreases the LT-HSC compartment. Altogether, these results show that BM resident macrophages are involved in the response of LT-HSCs to a 2 Gy-TBI and suggest that regulation of NO production can be used to modulate some deleterious effects of a TBI on LT-HSCs.
© 2022 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Figures




Similar articles
-
28Si total body irradiation injures bone marrow hematopoietic stem cells via induction of cellular apoptosis.Life Sci Space Res (Amst). 2017 May;13:39-44. doi: 10.1016/j.lssr.2017.03.003. Epub 2017 Apr 5. Life Sci Space Res (Amst). 2017. PMID: 28554508 Free PMC article.
-
Total body irradiation causes residual bone marrow injury by induction of persistent oxidative stress in murine hematopoietic stem cells.Free Radic Biol Med. 2010 Jan 15;48(2):348-56. doi: 10.1016/j.freeradbiomed.2009.11.005. Epub 2009 Dec 2. Free Radic Biol Med. 2010. PMID: 19925862 Free PMC article.
-
Mn(III) meso-tetrakis-(N-ethylpyridinium-2-yl) porphyrin mitigates total body irradiation-induced long-term bone marrow suppression.Free Radic Biol Med. 2011 Jul 1;51(1):30-7. doi: 10.1016/j.freeradbiomed.2011.04.016. Epub 2011 Apr 14. Free Radic Biol Med. 2011. PMID: 21565268 Free PMC article.
-
The maintenance mechanism of hematopoietic stem cell dormancy: role for a subset of macrophages.BMB Rep. 2023 Sep;56(9):482-487. doi: 10.5483/BMBRep.2023-0092. BMB Rep. 2023. PMID: 37574807 Free PMC article. Review.
-
Regulation of long-term repopulating hematopoietic stem cells by EPCR/PAR1 signaling.Ann N Y Acad Sci. 2016 Apr;1370(1):65-81. doi: 10.1111/nyas.13013. Epub 2016 Mar 1. Ann N Y Acad Sci. 2016. PMID: 26928241 Free PMC article. Review.
Cited by
-
EMP1 safeguards hematopoietic stem cells by suppressing sphingolipid metabolism and alleviating endoplasmic reticulum stress.Nat Commun. 2025 Jul 7;16(1):6247. doi: 10.1038/s41467-025-61552-0. Nat Commun. 2025. PMID: 40624017 Free PMC article.
-
Second bone marrow transplantation into regenerating hematopoiesis enhances reconstitution of immune system.Front Immunol. 2024 Jun 14;15:1405210. doi: 10.3389/fimmu.2024.1405210. eCollection 2024. Front Immunol. 2024. PMID: 38947315 Free PMC article.
-
Hematopoietic and stromal DMP1-Cre labeled cells form a unique niche in the bone marrow.Sci Rep. 2023 Dec 16;13(1):22403. doi: 10.1038/s41598-023-49713-x. Sci Rep. 2023. PMID: 38104230 Free PMC article.
References
-
- Rodrigues-Moreira S, Moreno SG, Ghinatti G, et al. . Low-dose irradiation promotes persistent oxidative stress and decreases self-renewal in hematopoietic stem cells. Cell Rep. 2017;20(13):3199-3211. - PubMed
-
- Lu Y, Hu M, Zhang Z, Qi Y, Wang J. The regulation of hematopoietic stem cell fate in the context of radiation. Radiat. Med. Prot. 2020;1(1):31-34.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

