A conditional glutamatergic synaptic vesicle marker for Drosophila
- PMID: 35100385
- PMCID: PMC8895992
- DOI: 10.1093/g3journal/jkab453
A conditional glutamatergic synaptic vesicle marker for Drosophila
Abstract
Glutamate is a principal neurotransmitter used extensively by the nervous systems of all vertebrate and invertebrate animals. It is primarily an excitatory neurotransmitter that has been implicated in nervous system development, as well as a myriad of brain functions from the simple transmission of information between neurons to more complex aspects of nervous system function including synaptic plasticity, learning, and memory. Identification of glutamatergic neurons and their sites of glutamate release are thus essential for understanding the mechanisms of neural circuit function and how information is processed to generate behavior. Here, we describe and characterize smFLAG-vGlut, a conditional marker of glutamatergic synaptic vesicles for the Drosophila model system. smFLAG-vGlut is validated for functionality, conditional expression, and specificity for glutamatergic neurons and synaptic vesicles. The utility of smFLAG-vGlut is demonstrated by glutamatergic neurotransmitter phenotyping of 26 different central complex neuron types of which nine were established to be glutamatergic. This illumination of glutamate neurotransmitter usage will enhance the modeling of central complex neural circuitry and thereby our understanding of information processing by this region of the fly brain. The use of smFLAG for glutamatergic neurotransmitter phenotyping and identification of glutamate release sites can be extended to any Drosophila neuron(s) represented by a binary transcription system driver.
Keywords: Drosophila; epitope tag; glutamatergic; synaptic vesicle; vGlut.
© The Author(s) 2022. Published by Oxford University Press on behalf of Genetics Society of America.
Figures
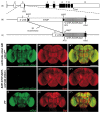
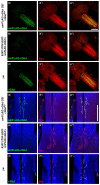
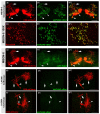

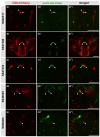
References
-
- Adams MD, Celniker SE, Holt RA, Evans CA, Gocayne JD, Amanatides PG, Scherer SE, Li PW, Hoskins RA, Galle RF, et al. The genome sequence of Drosophila melanogaster. Science. 2000;287:2185–2195. - PubMed
-
- Baker DA, Beckingham KM, Armstrong JD.. Functional dissection of the neural substrates for gravitaxic maze behavior in Drosophila melanogaster. J Comp Neurol. 2007;501(5):756–764. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
