Synthesis of Polycyclic Isoindolines via α-C-H/N-H Annulation of Alicyclic Amines
- PMID: 35100511
- PMCID: PMC9039734
- DOI: 10.1021/acs.orglett.2c00018
Synthesis of Polycyclic Isoindolines via α-C-H/N-H Annulation of Alicyclic Amines
Abstract
Relatively unstable cyclic imines, generated in situ from their corresponding alicyclic amines via oxidation of their lithium amides with simple ketone oxidants, engage aryllithium compounds containing a leaving group on an ortho-methylene functionality to provide polycyclic isoindolines in a single operation. The scope of this transformation includes pyrrolidine, piperidine, azepane, azocane, and piperazines.
Figures
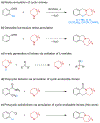
References
-
- Taylor RD; MacCoss M; Lawson ADG, Rings in Drugs. J. Med. Chem 2014, 57, 5845–5859 - PubMed
- Vitaku E; Smith DT; Njardarson JT, Analysis of the Structural Diversity, Substitution Patterns, and Frequency of Nitrogen Heterocycles among U.S. FDA Approved Pharmaceuticals. J. Med. Chem 2014, 57, 10257–10274. - PubMed
-
-
For a general overview of amine C–H bond functionalization, see
- Dutta S; Li B; Rickertsen DRL; Valles DA; Seidel D, C–H Bond Functionalization of Amines: A Graphical Overview of Diverse Methods. SynOpen 2021, 05, 173–228. - PMC - PubMed
-
Other selected reviews
- Campos KR, Direct sp3 C-H bond activation adjacent to nitrogen in heterocycles. Chem. Soc. Rev 2007, 36, 1069–1084 - PubMed
- Jazzar R; Hitce J; Renaudat A; Sofack-Kreutzer J; Baudoin O, Functionalization of Organic Molecules by Transition-Metal-Catalyzed C(sp3)-H Activation. Chem. Eur. J 2010, 16, 2654–2672 - PubMed
- Mitchell EA; Peschiulli A; Lefevre N; Meerpoel L; Maes BUW, Direct alpha-Functionalization of Saturated Cyclic Amines. Chem. Eur. J 2012, 18, 10092–10142 - PubMed
- Peng B; Maulide N, The Redox-Neutral Approach to C-H Functionalization. Chem. Eur. J 2013, 19, 13274–13287 - PubMed
- Girard SA; Knauber T; Li C-J, The Cross-Dehydrogenative Coupling of C sp3-H Bonds: A Versatile Strategy for C-C Bond Formations. Angew. Chem. Int. Ed 2014, 53, 74–100 - PubMed
- Haibach MC; Seidel D, C-H Bond Functionalization through Intramolecular Hydride Transfer. Angew. Chem. Int. Ed 2014, 53, 5010–5036 - PubMed
- Beatty JW; Stephenson CRJ, Amine Functionalization via Oxidative Photoredox Catalysis: Methodology Development and Complex Molecule Synthesis. Acc. Chem. Res 2015, 48, 1474–1484 - PMC - PubMed
- Cheng M-X; Yang S-D, Recent Advances in the Enantioselective Oxidative α-C–H Functionalization of Amines. Synlett 2017, 28, 159–174
- Chu JCK; Rovis T, Complementary Strategies for Directed C(sp3)−H Functionalization: A Comparison of Transition-Metal-Catalyzed Activation, Hydrogen Atom Transfer, and Carbene/Nitrene Transfer. Angew. Chem. Int. Ed 2018, 57, 62–101 - PMC - PubMed
- Stateman LM; Nakafuku KM; Nagib DA, Remote C–H Functionalization via Selective Hydrogen Atom Transfer. Synthesis 2018, 50, 1569–1586 - PMC - PubMed
- Edwards PM; Schafer LL, Early transition metal-catalyzed C–H alkylation: hydroaminoalkylation for Csp3–Csp3 bond formation in the synthesis of selectively substituted amines. Chem. Commun 2018, 54, 12543–12560 - PubMed
- Gonnard L; Guérinot A; Cossy J, Transition metal-catalyzed α-alkylation of amines by C(sp3)‒H bond activation. Tetrahedron 2019, 75, 145–163
- Liu S; Zhao Z; Wang Y, Construction of N-Heterocycles through Cyclization of Tertiary Amines. Chem. Eur. J 2019, 25, 2423–2441 - PubMed
- Antermite D; Bull JA, Transition Metal-Catalyzed Directed C(sp3)–H Functionalization of Saturated Heterocycles. Synthesis 2019, 51, 3171–3204
- Zhang T; Wu Y-H; Wang N-X; Xing Y, Advances in C(sp3)–H Bond Functionalization via Radical Processes. Synthesis 2019, 51, 4531–4548
- Trowbridge A; Walton SM; Gaunt MJ, New Strategies for the Transition-Metal Catalyzed Synthesis of Aliphatic Amines. Chem. Rev 2020, 120, 2613–2692 - PubMed
- Kapoor M; Singh A; Sharma K; Hua Hsu M, Site-Selective C(sp3)−H and C(sp2)−H Functionalization of Amines Using a Directing-Group-Guided Strategy. Adv. Synth. Catal 2020, 362, 4513–4542
- An X-D; Xiao J, Recent advances in hydride transfer-involved C(sp3)–H activation reactions. Org. Chem. Front 2021, 8, 1364–1383
- Basak S; Winfrey L; Kustiana BA; Melen RL; Morrill LC; Pulis AP, Electron deficient borane-mediated hydride abstraction in amines: stoichiometric and catalytic processes. Chem. Soc. Rev 2021, 50, 3720–3737 - PubMed
- Caplin MJ; Foley DJ, Emergent synthetic methods for the modular advancement of sp3-rich fragments. Chem. Sci 2021, 12, 4646–4660; - PMC - PubMed
- Ohno S; Miyoshi M; Murai K; Arisawa M, Non-Directed β- or γ-C(sp3)–H Functionalization of Saturated Nitrogen-Containing Heterocycles. Synthesis 2021, 53, 2947–2960
- He Y; Zheng Z; Yang J; Zhang X; Fan X, Recent advances in the functionalization of saturated cyclic amines. Org. Chem. Front 2021, 8, 4582–4606.
-
-
-
Selected recent examples of mechanistically diverse methods for amine C–H bond functionalization
- Ohmatsu K; Suzuki R; Furukawa Y; Sato M; Ooi T, Zwitterionic 1,2,3-Triazolium Amidate as a Catalyst for Photoinduced Hydrogen-Atom Transfer Radical Alkylation. ACS Catal 2020, 10, 2627–2632
- Roque JB; Kuroda Y; Jurczyk J; Xu L-P; Ham JS; Göttemann LT; Roberts CA; Adpressa D; Saurí J; Joyce LA; Musaev DG; Yeung CS; Sarpong R, C–C Cleavage Approach to C–H Functionalization of Saturated Aza-Cycles. ACS Catal. 2020, 10, 2929–2941 - PMC - PubMed
- Rand AW; Yin H; Xu L; Giacoboni J; Martin-Montero R; Romano C; Montgomery J; Martin R, Dual Catalytic Platform for Enabling sp3 α C–H Arylation and Alkylation of Benzamides. ACS Catal. 2020, 10, 4671–4676
- Liu W; Babl T; Röther A; Reiser O; Davies HML, Functionalization of Piperidine Derivatives for the Site-Selective and Stereoselective Synthesis of Positional Analogues of Methylphenidate. Chem. Eur. J 2020, 26, 4236–4241 - PMC - PubMed
- Verma P; Richter JM; Chekshin N; Qiao JX; Yu J-Q, Iridium(I)-Catalyzed α-C(sp3)–H Alkylation of Saturated Azacycles. J. Am. Chem. Soc 2020, 142, 5117–5125 - PMC - PubMed
- Walker MM; Koronkiewicz B; Chen S; Houk KN; Mayer JM; Ellman JA, Highly Diastereoselective Functionalization of Piperidines by Photoredox-Catalyzed α-Amino C–H Arylation and Epimerization. J. Am. Chem. Soc 2020, 142, 8194–8202 - PMC - PubMed
- Chen L; Yang Y; Liu L; Gao Q; Xu S, Iridium-Catalyzed Enantioselective α-C(sp3)–H Borylation of Azacycles. J. Am. Chem. Soc 2020, 142, 12062–12068 - PubMed
- Xu L-P; Roque JB; Sarpong R; Musaev DG, Reactivity and Selectivity Controlling Factors in the Pd/Dialkylbiarylphosphine-Catalyzed C–C Cleavage/Cross-Coupling of an N-Fused Bicyclo α-Hydroxy-β-Lactam. J. Am. Chem. Soc 2020, 142, 21140–21152 - PubMed
- Feng K; Quevedo RE; Kohrt JT; Oderinde MS; Reilly U; White MC, Late-stage oxidative C(sp3)–H methylation. Nature 2020, 580, 621–627 - PMC - PubMed
- Sarver PJ; Bacauanu V; Schultz DM; DiRocco DA; Lam Y.-h.; Sherer EC; MacMillan DWC, The merger of decatungstate and copper catalysis to enable aliphatic C(sp3)–H trifluoromethylation. Nat. Chem 2020, 12, 459–467 - PubMed
- McManus JB; Onuska NPR; Jeffreys MS; Goodwin NC; Nicewicz DA, Site-Selective C–H Alkylation of Piperazine Substrates via Organic Photoredox Catalysis. Org. Lett 2020, 22, 679–683 - PubMed
- Cao L; Zhao H; Tan Z; Guan R; Jiang H; Zhang M, Ruthenium-Catalyzed Hydrogen Evolution o-Aminoalkylation of Phenols with Cyclic Amines. Org. Lett 2020, 22, 4781–4785 - PubMed
- Chen Y; Wan H-L; Huang Y; Liu S; Wang F; Lu C; Nie J; Chen Z; Yang G; Ma C, B(C6F5)3-Catalyzed β-Functionalization of Pyrrolidines Using Isatins via Borrowing Hydrogen: Divergent Access to Substituted Pyrrolidines and Pyrroles. Org. Lett 2020, 22, 7797–7803 - PubMed
- Oeschger R; Su B; Yu I; Ehinger C; Romero E; He S; Hartwig J, Diverse functionalization of strong alkyl C–H bonds by undirected borylation. Science 2020, 368, 736–741 - PMC - PubMed
- Short MA; Blackburn JM; Roizen JL, Modifying Positional Selectivity in C–H Functionalization Reactions with Nitrogen-Centered Radicals: Generalizable Approaches to 1,6-Hydrogen-Atom Transfer Processes. Synlett 2020, 31, 102–116 - PMC - PubMed
- Kim Y; Heo J; Kim D; Chang S; Seo S, Ring-opening functionalizations of unstrained cyclic amines enabled by difluorocarbene transfer. Nat. Commun 2020, 11, 4761. - PMC - PubMed
- Trindade AF; Faulkner EL; Leach AG; Nelson A; Marsden SP, Fragment-oriented synthesis: β-elaboration of cyclic amine fragments using enecarbamates as platform intermediates. Chem. Commun 2020, 56, 8802–8805 - PubMed
- Holmberg-Douglas N; Choi Y; Aquila B; Huynh H; Nicewicz DA, β-Functionalization of Saturated Aza-Heterocycles Enabled by Organic Photoredox Catalysis. ACS Catal. 2021, 11, 3153–3158 - PMC - PubMed
- Yi M-J; Zhang H-X; Xiao T-F; Zhang J-H; Feng Z-T; Wei L-P; Xu G-Q; Xu P-F, Photoinduced Metal-Free α-C(sp3)–H Carbamoylation of Saturated Aza-Heterocycles via Rationally Designed Organic Photocatalyst. ACS Catal. 2021, 11, 3466–3472
- Aguilera EY; Sanford MS, Palladium-Mediated Cγ−H Functionalization of Alicyclic Amines. Angew. Chem. Int. Ed 2021, 60, 11227–11230 - PMC - PubMed
- Chang Y; Cao M; Chan JZ; Zhao C; Wang Y; Yang R; Wasa M, Enantioselective Synthesis of N-Alkylamines through β-Amino C–H Functionalization Promoted by Cooperative Actions of B(C6F5)3 and a Chiral Lewis Acid Co-Catalyst. J. Am. Chem. Soc 2021, 143, 2441–2455 - PMC - PubMed
- Yue W-J; Day CS; Martin R, Site-Selective Defluorinative sp3 C–H Alkylation of Secondary Amides. J. Am. Chem. Soc 2021, 143, 6395–6400 - PubMed
- Koperniku A; Schafer LL, Zirconium Catalyzed Hydroaminoalkylation for the Synthesis of α-Arylated Amines and N-Heterocycles. Chem. Eur. J 2021, 27, 6334–6339. - PubMed
-
-
-
For a comprehensive review, see
- Chen W; Seidel D, Condensation-Based Methods for the C–H Bond Functionalization of Amines. Synthesis 2021, 53, 3869–3908. Selected examples of redox-annulations - PMC - PubMed
- Zhang C; De CK; Mal R; Seidel D, alpha-Amination of Nitrogen Heterocycles: Ring-Fused Aminals. J. Am. Chem. Soc 2008, 130, 416–417 - PubMed
- Zheng L; Yang F; Dang Q; Bai X, A Cascade Reaction with Iminium Ion Isomerization as the Key Step Leading to Tetrahydropyrimido[4,5-d]pyrimidines. Org. Lett 2008, 10, 889–892 - PubMed
- Zhang C; Das D; Seidel D, Azomethine ylide annulations: facile access to polycyclic ring systems. Chem. Sci 2011, 2, 233–236
- Dieckmann A; Richers MT; Platonova AY; Zhang C; Seidel D; Houk KN, Metal-Free α-Amination of Secondary Amines: Computational and Experimental Evidence for Azaquinone Methide and Azomethine Ylide Intermediates. J. Org. Chem 2013, 78, 4132–4144 - PMC - PubMed
- Richers MT; Breugst M; Platonova AY; Ullrich A; Dieckmann A; Houk KN; Seidel D, Redox-Neutral α-Oxygenation of Amines: Reaction Development and Elucidation of the Mechanism. J. Am. Chem. Soc 2014, 136, 6123–6135 - PMC - PubMed
- Kang Y; Chen W; Breugst M; Seidel D, Asymmetric Redox-Annulation of Cyclic Amines. J. Org. Chem 2015, 80, 9628–9640 - PMC - PubMed
- Chen W; Seidel D, Redox-Annulation of Cyclic Amines and β-Ketoaldehydes. Org. Lett 2016, 18, 1024–1027 - PMC - PubMed
- Li J; Fu Y; Qin C; Yu Y; Li H; Wang W, Asymmetric synthesis of isoquinolinonaphthyridines catalyzed by a chiral Bronsted acid. Org. Biomol. Chem 2017, 15, 6474–6477 - PubMed
- Liu Y; Wu J; Jin Z; Jiang H, Synthesis of 1,2-Fused Bicyclic Imidazolidin-4-ones by Redox-Neutral Cyclization Reaction of Cyclic Amines and α-Ketoamides. Synlett 2018, 29, 1061–1064
- Paul A; Chandak HS; Ma L; Seidel D, Redox-Annulations of Cyclic Amines with ortho-Cyanomethylbenzaldehydes. Org. Lett 2020, 22, 976–980 - PMC - PubMed
- Rickertsen DRL; Ma L; Paul A; Abboud KA; Seidel D, Traceless Redox-Annulations of Alicyclic Amines. SynOpen 2020, 04, 123–131. - PMC - PubMed
-
Grants and funding
LinkOut - more resources
Full Text Sources

