The kidney releases a nonpolymerizing form of uromodulin in the urine and circulation that retains the external hydrophobic patch domain
- PMID: 35100812
- PMCID: PMC8934678
- DOI: 10.1152/ajprenal.00322.2021
The kidney releases a nonpolymerizing form of uromodulin in the urine and circulation that retains the external hydrophobic patch domain
Abstract
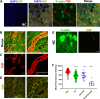
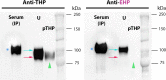
Uromodulin [Tamm-Horsfall protein (THP)] is a glycoprotein uniquely produced in the kidney. It is released by cells of the thick ascending limbs apically in the urine and basolaterally in the renal interstitium and systemic circulation. Processing of mature urinary THP, which polymerizes into supramolecular filaments, requires cleavage of an external hydrophobic patch (EHP) at the COOH-terminus. However, THP in the circulation is not polymerized, and it remains unclear if nonaggregated forms of THP exist natively in the urine. We propose that an alternative processing path, which retains the EHP domain, can lead to a nonpolymerizing form of THP. We generated an antibody that specifically recognizes THP with retained EHP (THP + EHP) and established its presence in the urine in a nonpolymerized native state. Proteomic characterization of urinary THP + EHP revealed its COOH-terminus ending at F617. In the human kidney, THP + EHP was detected in thick ascending limb cells and less strongly in the renal parenchyma. Using immunoprecipitation followed by proteomic sequencing and immunoblot analysis, we then demonstrated that serum THP has also retained EHP. In a small cohort of patients at risk for acute kidney injury, admission urinary THP + EHP was significantly lower in patients who subsequently developed acute kidney injury during hospitalization. Our findings uncover novel insights into uromodulin biology by establishing the presence of an alternative path for cellular processing, which could explain the release of nonpolymerizing THP in the circulation. Larger studies are needed to establish the utility of urinary THP + EHP as a sensitive biomarker of kidney health and susceptibility to injury.NEW & NOTEWORTHY In this work, we discovered and characterized a novel form of uromodulin that does not polymerize because it retains an external hydrophobic patch at the COOH-terminus. These findings establish an alternative form of cellular processing of this protein and elucidate new aspects of its biology. We also provide evidence suggesting that measuring urinary nonpolymerizing uromodulin could be a promising assay to assess the risk of acute kidney injury.
Keywords: Tamm-Horsfall protein; kidney injury; thick ascending limb; uromodulin.
Conflict of interest statement
The authors have applied for a patent to detect the nonpolymerizing form of THP using the reagents and ELISA tools that they developed.
Figures






References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

