Evidence for Horizontal and Vertical Transmission of Mtr-Mediated Extracellular Electron Transfer among the Bacteria
- PMID: 35100867
- PMCID: PMC8805035
- DOI: 10.1128/mbio.02904-21
Evidence for Horizontal and Vertical Transmission of Mtr-Mediated Extracellular Electron Transfer among the Bacteria
Abstract
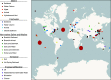
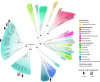
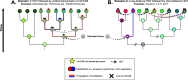
Some bacteria and archaea have evolved the means to use extracellular electron donors and acceptors for energy metabolism, a phenomenon broadly known as extracellular electron transfer (EET). One such EET mechanism is the transmembrane electron conduit MtrCAB, which has been shown to transfer electrons derived from metabolic substrates to electron acceptors, like Fe(III) and Mn(IV) oxides, outside the cell. Although most studies of MtrCAB-mediated EET have been conducted in Shewanella oneidensis MR-1, recent investigations in Vibrio and Aeromonas species have revealed that the electron-donating proteins that support MtrCAB in Shewanella are not as representative as previously thought. This begs the question of how widespread the capacity for MtrCAB-mediated EET is, the changes it has accrued in different lineages, and where these lineages persist today. Here, we employed a phylogenetic and comparative genomics approach to identify the MtrCAB system across all domains of life. We found mtrCAB in the genomes of numerous diverse Bacteria from a wide range of environments, and the patterns therein strongly suggest that mtrCAB was distributed through both horizontal and subsequent vertical transmission, and with some cases indicating downstream modular diversification of both its core and accessory components. Our data point to an emerging evolutionary story about metal-oxidizing and -reducing metabolism, demonstrates that this capacity for EET has broad relevance to a diversity of taxa and the biogeochemical cycles they drive, and lays the foundation for further studies to shed light on how this mechanism may have coevolved with Earth's redox landscape. IMPORTANCE While many metabolisms make use of soluble, cell-permeable substrates like oxygen or hydrogen, there are other substrates, like iron or manganese, that cannot be brought into the cell. Some bacteria and archaea have evolved the means to directly "plug in" to such environmental electron reservoirs in a process known as extracellular electron transfer (EET), making them powerful agents of biogeochemical change and promising vehicles for bioremediation and alternative energy. Yet the diversity, distribution, and evolution of EET mechanisms are poorly constrained. Here, we present findings showing that the genes encoding one such EET system (mtrCAB) are present in a broad diversity of bacteria found in a wide range of environments, emphasizing the ubiquity and potential impact of EET in our biosphere. Our results suggest that these genes have been disseminated largely through horizontal transfer, and the changes they have accrued in these lineages potentially reflect adaptations to changing environments.
Keywords: Shewanella; electron transport; evolution; gene transfer; iron oxidizers; iron reduction; lithoautotrophic metabolism; phylogenetic analysis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Aromokeye DA, Kulkarni AC, Elvert M, Wegener G, Henkel S, Coffinet S, Eickhorst T, Oni OE, Richter-Heitmann T, Schnakenberg A, Taubner H, Wunder L, Yin X, Zhu Q, Hinrichs K-U, Kasten S, Friedrich MW. 2019. Rates and microbial players of iron-driven anaerobic oxidation of methane in methanic marine sediments. Front Microbiol 10:3041. doi: 10.3389/fmicb.2019.03041. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

