Circulating tumor cell gene expression and plasma AR gene copy number as biomarkers for castration-resistant prostate cancer patients treated with cabazitaxel
- PMID: 35101049
- PMCID: PMC8805338
- DOI: 10.1186/s12916-022-02244-0
Circulating tumor cell gene expression and plasma AR gene copy number as biomarkers for castration-resistant prostate cancer patients treated with cabazitaxel
Abstract
Background: Cabazitaxel improves overall survival (OS) in metastatic castration-resistant prostate cancer (mCRPC) patients progressing after docetaxel. In this prospective study, we evaluated the prognostic role of CTC gene expression on cabazitaxel-treated patients and its association with plasma androgen receptor (AR) copy number (CN).
Methods: Patients receiving cabazitaxel 20 or 25 mg/sqm for mCRPC were enrolled. Digital PCR was performed to assess plasma AR CN status. CTC enrichment was assessed using the AdnaTest EMT-2/StemCell kit. CTC expression analyses were performed for 17 genes. Data are expressed as hazard ratio (HR) or odds ratio (OR) and 95% CI.
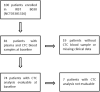
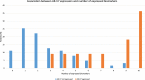
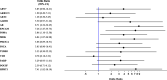
Results: Seventy-four patients were fully evaluable. CTC expression of AR-V7 (HR=2.52, 1.24-5.12, p=0.011), AKR1C3 (HR=2.01, 1.06-3.81, p=0.031), AR (HR=2.70, 1.46-5.01, p=0.002), EPCAM (HR=3.75, 2.10-6.71, p< 0.0001), PSMA (HR=2.09, 1.19-3.66, p=0.01), MDK (HR=3.35, 1.83-6.13, p< 0.0001), and HPRT1 (HR=2.46, 1.44-4.18, p=0.0009) was significantly associated with OS. ALDH1 (OR=5.50, 0.97-31.22, p=0.05), AR (OR=8.71, 2.32-32.25, p=0.001), EPCAM (OR=7.26, 1.47-35.73, p=0.015), PSMA (OR=3.86, 1.10-13.50, p=0.035), MDK (OR=6.84, 1.87-24.98, p=0.004), and HPRT1 (OR=7.41, 1.82-30.19, p=0.005) expression was associated with early PD. AR CN status was significantly correlated with AR-V7 (p=0.05), EPCAM (p=0.02), and MDK (p=0.002) expression. In multivariable model, EPCAM and HPRT1 CTC expression, plasma AR CN gain, ECOG PS=2, and liver metastases and PSA were independently associated with poorer OS. In patients treated with cabazitaxel 20 mg/sqm, median OS was shorter in AR-V7 positive than negative patients (6.6 versus 14 months, HR=3.46, 1.47-8.17], p=0.004).
Conclusions: Baseline CTC biomarkers may be prognosticators for cabazitaxel-treated mCRPC patients. Cabazitaxel at lower (20 mg/sqm) dose was associated with poorer outcomes in AR-V7 positive patients compared to AR-V7 negative patients in a post hoc subgroup analysis.
Trial registration: Clinicaltrials.gov NCT03381326 . Retrospectively registered on 18 December 2017.
Keywords: AR copy number; AR-V7; CTC; Cabazitaxel; mCRPC.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
The Impact of Circulating Tumor Cell HOXB13 RNA Detection in Men with Metastatic Castration-Resistant Prostate Cancer (mCRPC) Treated with Abiraterone or Enzalutamide.Clin Cancer Res. 2024 Mar 15;30(6):1152-1159. doi: 10.1158/1078-0432.CCR-23-3017. Clin Cancer Res. 2024. PMID: 38236581 Free PMC article.
-
Sequencing impact and prognostic factors in metastatic castration-resistant prostate cancer patients treated with cabazitaxel: A systematic review and meta-analysis.Urol Oncol. 2023 Apr;41(4):177-191. doi: 10.1016/j.urolonc.2022.06.018. Epub 2022 Aug 13. Urol Oncol. 2023. PMID: 35970698
-
Circulating Tumor Cell Count and Overall Survival in Patients With Metastatic Hormone-Sensitive Prostate Cancer.JAMA Netw Open. 2024 Oct 1;7(10):e2437871. doi: 10.1001/jamanetworkopen.2024.37871. JAMA Netw Open. 2024. PMID: 39374015 Free PMC article. Clinical Trial.
-
Circulating Biomarkers Predictive of Treatment Response in Patients with Hormone-sensitive or Castration-resistant Metastatic Prostate Cancer: A Systematic Review.Eur Urol Oncol. 2024 Dec;7(6):1228-1245. doi: 10.1016/j.euo.2024.05.003. Epub 2024 May 31. Eur Urol Oncol. 2024. PMID: 38824003
-
Efficacy of Cabazitaxel in Castration-resistant Prostate Cancer Is Independent of the Presence of AR-V7 in Circulating Tumor Cells.Eur Urol. 2015 Dec;68(6):939-45. doi: 10.1016/j.eururo.2015.07.007. Epub 2015 Jul 15. Eur Urol. 2015. PMID: 26188394 Clinical Trial.
Cited by
-
Integrative single-cell and bulk transcriptomes analyses reveals heterogeneity of serine-glycine-one-carbon metabolism with distinct prognoses and therapeutic vulnerabilities in HNSCC.Int J Oral Sci. 2024 Jun 17;16(1):44. doi: 10.1038/s41368-024-00310-2. Int J Oral Sci. 2024. PMID: 38886346 Free PMC article.
-
Plasma Exosomal AKR1C3 mRNA Expression Is a Predictive and Prognostic Biomarker in Patients with Metastatic Castration-Resistant Prostate Cancer.Oncologist. 2022 Nov 3;27(11):e870-e877. doi: 10.1093/oncolo/oyac177. Oncologist. 2022. PMID: 36067250 Free PMC article.
-
Advances in research regarding epithelial-mesenchymal transition and prostate cancer.Front Cell Dev Biol. 2025 May 30;13:1583255. doi: 10.3389/fcell.2025.1583255. eCollection 2025. Front Cell Dev Biol. 2025. PMID: 40519259 Free PMC article. Review.
-
Longitudinal assessment of plasma androgen receptor copy number predicts overall survival in subsequent treatment lines in castration-resistant prostate cancer: analysis from a prospective trial.ESMO Open. 2023 Dec;8(6):102036. doi: 10.1016/j.esmoop.2023.102036. Epub 2023 Oct 20. ESMO Open. 2023. PMID: 37866028 Free PMC article.
-
Changing metastatic patterns associate with dynamics of circulating tumor DNA in metastatic castration-resistant prostate cancer.Oncologist. 2025 May 8;30(5):oyaf107. doi: 10.1093/oncolo/oyaf107. Oncologist. 2025. PMID: 40377440 Free PMC article.
References
-
- Nuhn P, De Bono JS, Fizazi K, Freedland SJ, Grilli M, Kantoff PW, Sonpavde G, Sternberg CN, Yegnasubramanian S, Antonarakis ES. Update on systemic prostate cancer therapies: management of metastatic castration-resistant prostate cancer in the era of precision oncology. Eur Urol. 2019;75(1):88–99. doi: 10.1016/j.eururo.2018.03.028. - DOI - PubMed
-
- Brighi N, Conteduca V, Lolli C, Gurioli G, Schepisi G, Palleschi M, Mariotti M, Casadei C, De Giorgi U. The cyclin-dependent kinases pathway as a target for prostate cancer treatment: rationale and future perspectives. Crit Rev Oncol Hematol. 2021;157:103199. doi: 10.1016/j.critrevonc.2020.103199. - DOI - PubMed
-
- Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, Oudard S, Théodore C, James ND, Turesson I, Rosenthal MA, Eisenberger MA, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351(15):1502–1512. doi: 10.1056/NEJMoa040720. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

