Worldwide experience of homozygous familial hypercholesterolaemia: retrospective cohort study
- PMID: 35101175
- PMCID: PMC10544712
- DOI: 10.1016/S0140-6736(21)02001-8
Worldwide experience of homozygous familial hypercholesterolaemia: retrospective cohort study
Abstract
Background: Homozygous familial hypercholesterolaemia (HoFH) is a rare inherited disorder resulting in extremely elevated low-density lipoprotein cholesterol levels and premature atherosclerotic cardiovascular disease (ASCVD). Current guidance about its management and prognosis stems from small studies, mostly from high-income countries. The objective of this study was to assess the clinical and genetic characteristics, as well as the impact, of current practice on health outcomes of HoFH patients globally.
Methods: The HoFH International Clinical Collaborators registry collected data on patients with a clinical, or genetic, or both, diagnosis of HoFH using a retrospective cohort study design. This trial is registered with ClinicalTrials.gov, NCT04815005.
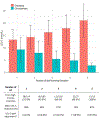
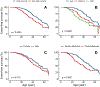
Findings: Overall, 751 patients from 38 countries were included, with 565 (75%) reporting biallelic pathogenic variants. The median age of diagnosis was 12·0 years (IQR 5·5-27·0) years. Of the 751 patients, 389 (52%) were female and 362 (48%) were male. Race was reported for 527 patients; 338 (64%) patients were White, 121 (23%) were Asian, and 68 (13%) were Black or mixed race. The major manifestations of ASCVD or aortic stenosis were already present in 65 (9%) of patients at diagnosis of HoFH. Globally, pretreatment LDL cholesterol levels were 14·7 mmol/L (IQR 11·6-18·4). Among patients with detailed therapeutic information, 491 (92%) of 534 received statins, 342 (64%) of 534 received ezetimibe, and 243 (39%) of 621 received lipoprotein apheresis. On-treatment LDL cholesterol levels were lower in high-income countries (3·93 mmol/L, IQR 2·6-5·8) versus non-high-income countries (9·3 mmol/L, 6·7-12·7), with greater use of three or more lipid-lowering therapies (LLT; high-income 66% vs non-high-income 24%) and consequently more patients attaining guideline-recommended LDL cholesterol goals (high-income 21% vs non-high-income 3%). A first major adverse cardiovascular event occurred a decade earlier in non-high-income countries, at a median age of 24·5 years (IQR 17·0-34·5) versus 37·0 years (29·0-49·0) in high-income countries (adjusted hazard ratio 1·64, 95% CI 1·13-2·38).
Interpretation: Worldwide, patients with HoFH are diagnosed too late, undertreated, and at high premature ASCVD risk. Greater use of multi-LLT regimens is associated with lower LDL cholesterol levels and better outcomes. Significant global disparities exist in treatment regimens, control of LDL cholesterol levels, and cardiovascular event-free survival, which demands a critical re-evaluation of global health policy to reduce inequalities and improve outcomes for all patients with HoFH.
Funding: Amsterdam University Medical Centers, Location Academic Medical Center; Perelman School of Medicine at the University of Pennsylvania; and European Atherosclerosis Society.
Copyright © 2022 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests SB declares no competing interests. DJB reports research grants from Amgen, Amryt, AstraZeneca, Sanofi, and Regeneron; lecture fees and personal fees from Amgen, Sanofi-Aventis and Novartis; participation in advisory board for Amryt (Chair of the LOWER study steering committee); and being member of the executive committee of the Lipid and Atherosclerosis Society of South Africa. MC reports institutional support for the conduction of clinical trials from Regeneron Pharmaceuticals, Akcea, and Regenxbio; consulting fees from Amryt Pharma; and support from NIH/NHLBI grant (P01HL059407). TF reports personal fees from Novartis, Sanofi, and Amgen; and that he was partly supported by the Ministry of Health, Czech Republic, grant number NU20-02-00261. MH-S reports research grants from Recordati and Kaneka; personal fees from Amgen, Astellas, Recordati, Merck Sharp & Dohme, and Sanofi; being on the advisory board for New Amsterdam Pharma and Medicine Company and Scilence Therapeutics; being chairperson Primary Hyperlipidemia, Research on Measures against Intractable Diseases by the Japanese Ministry of Health, Labor, and Welfare; being chairperson of the Working Group by Japan Atherosclerosis Society for Making Guidance of Familial Hypercholesterolemia; and owning stock options of Liid Pharma. MLH reports lecture fees from Sanofi, outside the submitted work. GKH reports research grants from the Netherlands Organization for Scientific Research (vidi 016.156.445), CardioVascular Research Initiative, EU, and the Klinkerpad fonds; institutional research support from Aegerion, Amgen, AstraZeneca, Eli Lilly, Genzyme, Ionis, Kowa, Pfizer, Regeneron, Roche, Sanofi, and The Medicines Company; speaker's bureau and consulting fees from Amgen, Aegerion, Sanofi, and Regeneron until April 2019 (fees paid to the academic institution); and part-time employment at Novo Nordisk, Denmark since April, 2019. FJR reports consulting fees, lecturing fees, and advisory board fees from Amgen, Sanofi-Aventis, Regeneron, Novartis and Lib Therapeutics outside the submitted work; and being member of the International Atherosclerosis Society. KKR reports institutional research grants from Amgen, Sanofi, Daiichi Sankyo, Regeneron, and Pfizer; consulting fees and lecturing fees from Amgen, Sanofi, Novartis, Pfizer, AstraZeneca, Boehringer Ingelheim, Novo Nordisk, Kowa, Silence Therapeutics, New Amsterdam, Esperion, Daiichi Sankyo, Bayer, Abbott, Resverlogix, Medicines Company, Eli Lilly, Algorithm, Merck, Sharp & Dohme, AbbVie, and Viatris, outside the submitted work. HS reports research grants from Amgen, Merck, Sharp & Dohme, Synageva, Amryt, Alexion, and Akcea; consulting fees from Amgen, Alexion, Daiichi Sankyo, Pfizer, and Akcea; and speaker fees from Amgen, Daiichi Sankyo, Sanofi, and Akcea. TRT declares no competing interests. AJV-V reports participation in research grants to Imperial College London or European Atherosclerosis Society, or both, from Pfizer, Amgen, Merck Sharp & Dohme, Sanofi-Aventis, Daiichi Sankyo, and Regeneron; personal fees for consulting from Bayer and Regeneron; and honoraria for lectures from Amgen, Mylan, and Akcea; outside the submitted work.
Figures
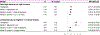
Comment in
-
Homozygous familiar hypercholesterolemia: still a long way to go.Lancet. 2022 Feb 19;399(10326):696-697. doi: 10.1016/S0140-6736(21)02223-6. Epub 2022 Jan 28. Lancet. 2022. PMID: 35101174 No abstract available.
References
-
- Cuchel M, Bruckert E, Ginsberg HN, et al. Homozygous familial hypercholesterolaemia: new insights and guidance for clinicians to improve detection and clinical management. A position paper from the Consensus Panel on Familial Hypercholesterolaemia of the European Atherosclerosis Society. Eur Heart J 2014; 35: 2146–57. - PMC - PubMed
-
- Sjouke B, Kusters DM, Kindt I, et al. Homozygous autosomal dominant hypercholesterolaemia in the Netherlands: Prevalence, genotype-phenotype relationship, and clinical outcome. Eur Heart J 2015; 36: 560–5. - PubMed
-
- Hu P, Dharmayat KI, Stevens CAT, et al. Prevalence of Familial Hypercholesterolemia among the General Population and Patients with Atherosclerotic Cardiovascular Disease: A Systematic Review and Meta-Analysis. Circulation 2020; 141: 1742–59. - PubMed
-
- Beheshti SO, Madsen CM, Varbo A, Nordestgaard BG. Worldwide Prevalence of Familial Hypercholesterolemia: Meta-Analyses of 11 Million Subjects. J Am Coll Cardiol 2020; 75: 2553–66. - PubMed

