On the management of maternal pushing during the second stage of labor: a biomechanical study considering passive tissue fatigue damage accumulation
- PMID: 35101408
- PMCID: PMC9308631
- DOI: 10.1016/j.ajog.2022.01.023
On the management of maternal pushing during the second stage of labor: a biomechanical study considering passive tissue fatigue damage accumulation
Abstract
Background: During the second stage of labor, the maternal pelvic floor muscles undergo repetitive stretch loading as uterine contractions and strenuous maternal pushes combined to expel the fetus, and it is not uncommon that these muscles sustain a partial or complete rupture. It has recently been demonstrated that soft tissues, including the anterior cruciate ligament and connective tissue in sheep pelvic floor muscle, can accumulate damage under repetitive physiological (submaximal) loads. It is well known to material scientists that this damage accumulation can not only decrease tissue resistance to stretch but also result in a partial or complete structural failure. Thus, we wondered whether certain maternal pushing patterns (in terms of frequency and duration of each push) could increase the risk of excessive damage accumulation in the pelvic floor tissue, thereby inadvertently contributing to the development of pelvic floor muscle injury.
Objective: This study aimed to determine which labor management practices (spontaneous vs directed pushing) are less prone to accumulate damage in the pelvic floor muscles during the second stage of labor and find the optimum approach in terms of minimizing the risk of pelvic floor muscle injury.
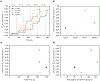
Study design: We developed a biomechanical model for the expulsive phase of the second stage of labor that includes the ability to measure the damage accumulation because of repetitive physiological submaximal loads. We performed 4 simulations of the second stage of labor, reflecting a directed pushing technique and 3 alternatives for spontaneous pushing.
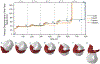
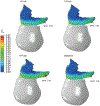
Results: The finite element model predicted that the origin of the pubovisceral muscle accumulates the most damage and so it is the most likely place for a tear to develop. This result was independent of the pushing pattern. Performing 3 maternal pushes per contraction, with each push lasting 5 seconds, caused less damage and seemed the best approach. The directed pushing technique (3 pushes per contraction, with each push lasting 10 seconds) did not reduce the duration of the second stage of labor and caused higher damage accumulation.
Conclusion: The frequency and duration of the maternal pushes influenced the damage accumulation in the passive tissues of the pelvic floor muscles, indicating that it can influence the prevalence of pelvic floor muscle injuries. Our results suggested that the maternal pushes should not last longer than 5 seconds and that the duration of active pushing is a better measurement than the total duration of the second stage of labor. Hopefully, this research will help to shed new light on the best practices needed to improve the experience of labor for women.
Keywords: finite element method; low-cycle fatigue failure; management of labor; maternal pushes; pelvic floor muscle; second stage of labor; vaginal delivery.
Copyright © 2022 Elsevier Inc. All rights reserved.
Figures




Similar articles
-
Biomechanical analyses of the efficacy of patterns of maternal effort on second-stage progress.Obstet Gynecol. 2009 Apr;113(4):873-880. doi: 10.1097/AOG.0b013e31819c82e1. Obstet Gynecol. 2009. PMID: 19305333 Free PMC article.
-
Soft-tissue dystocia due to paradoxical contraction of the levator ani as a cause of prolonged second stage: concept, diagnosis, and potential treatment.Am J Obstet Gynecol. 2024 Mar;230(3S):S856-S864. doi: 10.1016/j.ajog.2022.12.323. Epub 2023 Jul 20. Am J Obstet Gynecol. 2024. PMID: 38462259 Review.
-
Investigating the birth-related caudal maternal pelvic floor muscle injury: The consequences of low cycle fatigue damage.J Mech Behav Biomed Mater. 2020 Oct;110:103956. doi: 10.1016/j.jmbbm.2020.103956. Epub 2020 Jul 9. J Mech Behav Biomed Mater. 2020. PMID: 32957249 Free PMC article.
-
Pelvic floor muscle injury during a difficult labor. Can tissue fatigue damage play a role?Int Urogynecol J. 2022 Feb;33(2):211-220. doi: 10.1007/s00192-021-05012-5. Epub 2021 Nov 16. Int Urogynecol J. 2022. PMID: 34783861 Free PMC article.
-
Comparison of maternal and fetal outcomes between delayed and immediate pushing in the second stage of vaginal delivery: systematic review and meta-analysis of randomized controlled trials.Arch Gynecol Obstet. 2021 Feb;303(2):481-499. doi: 10.1007/s00404-020-05814-w. Epub 2020 Sep 29. Arch Gynecol Obstet. 2021. PMID: 32990782
Cited by
-
Midwives' practices on perineal protection and episiotomy decision-making: A qualitative and descriptive study.Eur J Midwifery. 2024 May 10;8. doi: 10.18332/ejm/174126. eCollection 2024. Eur J Midwifery. 2024. PMID: 38736456 Free PMC article.
References
-
- Andrade FXC, César de Sá JMA, Andrade Pires FM. A ductile damage nonlocal model of integral-type at finite strains: formulation and numerical issues. Int J Damage Mech. 2011;20(4):515–557. doi:10.1177/1056789510386850 - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

