NRF2: KEAPing Tumors Protected
- PMID: 35101864
- PMCID: PMC8904278
- DOI: 10.1158/2159-8290.CD-21-0922
NRF2: KEAPing Tumors Protected
Abstract
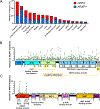
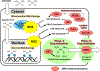
The Kelch-like ECH-associated protein 1 (KEAP1)/nuclear factor erythroid 2-related factor 2 (NRF2) pathway plays a physiologic protective role against xenobiotics and reactive oxygen species. However, activation of NRF2 provides a powerful selective advantage for tumors by rewiring metabolism to enhance proliferation, suppress various forms of stress, and promote immune evasion. Genetic, epigenetic, and posttranslational alterations that activate the KEAP1/NRF2 pathway are found in multiple solid tumors. Emerging clinical data highlight that alterations in this pathway result in resistance to multiple therapies. Here, we provide an overview of how dysregulation of the KEAP1/NRF2 pathway in cancer contributes to several hallmarks of cancer that promote tumorigenesis and lead to treatment resistance.
Significance: Alterations in the KEAP1/NRF2 pathway are found in multiple cancer types. Activation of NRF2 leads to metabolic rewiring of tumors that promote tumor initiation and progression. Here we present the known alterations that lead to NRF2 activation in cancer, the mechanisms in which NRF2 activation promotes tumors, and the therapeutic implications of NRF2 activation.
©2022 American Association for Cancer Research.
Conflict of interest statement
R.P, M.H and A.M.Z. have no conflict of interests.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

