Somatic and Germline Genomic Alterations in Very Young Women with Breast Cancer
- PMID: 35101884
- PMCID: PMC9359721
- DOI: 10.1158/1078-0432.CCR-21-2572
Somatic and Germline Genomic Alterations in Very Young Women with Breast Cancer
Abstract
Purpose: Young age at breast cancer diagnosis correlates with unfavorable clinicopathologic features and worse outcomes compared with older women. Understanding biological differences between breast tumors in young versus older women may lead to better therapeutic approaches for younger patients.
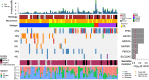
Experimental design: We identified 100 patients ≤35 years old at nonmetastatic breast cancer diagnosis who participated in the prospective Young Women's Breast Cancer Study cohort. Tumors were assigned a surrogate intrinsic subtype based on receptor status and grade. Whole-exome sequencing of tumor and germline samples was performed. Genomic alterations were compared with older women (≥45 years old) in The Cancer Genome Atlas, according to intrinsic subtype.
Results: Ninety-three tumors from 92 patients were successfully sequenced. Median age was 32.5 years; 52.7% of tumors were hormone receptor-positive/HER2-negative, 28.0% HER2-positive, and 16.1% triple-negative. Comparison of young to older women (median age 61 years) with luminal A tumors (N = 28 young women) revealed three significant differences: PIK3CA alterations were more common in older patients, whereas GATA3 and ARID1A alterations were more common in young patients. No significant genomic differences were found comparing age groups in other intrinsic subtypes. Twenty-two patients (23.9%) in the Young Women's Study cohort carried a pathogenic germline variant, most commonly (13 patients, 14.1%) in BRCA1/2.
Conclusions: Somatic alterations in three genes (PIK3CA, GATA3, and ARID1A) occur at different frequencies in young versus older women with luminal A breast cancer. Additional investigation of these genes and associated pathways could delineate biological susceptibilities and improve treatment options for young patients with breast cancer. See related commentary by Yehia and Eng, p. 2209.
©2022 The Authors; Published by the American Association for Cancer Research.
Figures

Comment in
-
One Size Does Not Fit All: Breast Cancer in Young Women.Clin Cancer Res. 2022 Jun 1;28(11):2209-2210. doi: 10.1158/1078-0432.CCR-22-0352. Clin Cancer Res. 2022. PMID: 35320364
References
-
- Society AC. American cancer society: breast cancer facts and figures 2019–2020. <https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-...>.
-
- Anders CK, Hsu DS, Broadwater G, Acharya CR, Foekens JA, Zhang Y, et al. Young age at diagnosis correlates with worse prognosis and defines a subset of breast cancers with shared patterns of gene expression. J Clin Oncol 2008;26:3324–30. - PubMed
-
- Partridge AH, Hughes ME, Warner ET, Ottesen RA, Wong YN, Edge SB, et al. Subtype-dependent relationship between young age at diagnosis and breast cancer survival. J Clin Oncol 2016;34:3308–14. - PubMed
-
- Johansson ALV, Trewin CB, Hjerkind KV, Ellingjord-Dale M, Johannesen TB, Ursin G. Breast cancer-specific survival by clinical subtype after 7 years follow-up of young and elderly women in a nationwide cohort. Int J Cancer 2019;144:1251–61. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

