Tumor mutation burden for predicting immune checkpoint blockade response: the more, the better
- PMID: 35101940
- PMCID: PMC8804687
- DOI: 10.1136/jitc-2021-003087
Tumor mutation burden for predicting immune checkpoint blockade response: the more, the better
Abstract
Background: Recently, the US Food and Drug Administration (FDA) has approved immune checkpoint blockade (ICB) for treating cancer patients with tumor mutation burden (TMB) >10 mutations/megabase (mut/Mb). However, high TMB (TMB-H) defined by >10 mut/Mb fails to predict ICB response across different cancer types, which has raised serious concerns on the current FDA approval. Thus, to better implement TMB as a robust biomarker of ICB response, an optimal and generalizable TMB cut-off within and across cancer types must be addressed as soon as possible.
Methods: Using Morris's and Kurzrock's cohorts (n=1662 and 102), we exhaustively tested all possible TMB cut-offs for predicting ICB treatment outcomes in 10 cancer types. The bootstrap method was applied to generate 10,000 randomly resampled cohorts using original cohorts to measure the reproducibility of TMB cut-off. ICB treatment outcomes were analyzed by overall survival, progression-free survival and objective response rate.
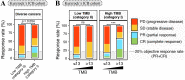
Results: No universally valid TMB cut-off was available for all cancer types. Only in cancer types with higher TMB (category I), such as melanoma, colorectal cancer, bladder cancer, and non-small cell lung cancer, the associations between TMB-H and ICB treatment outcomes were less affected by TMB cut-off selection. Moreover, high TMB (category I) cancer types shared a wide range of TMB cut-offs and a universally optimal TMB cut-off of 13 mut/Mb for predicting favorable ICB outcomes. In contrast, low TMB (category II) cancer types, for which the prognostic associations were sensitive to TMB cut-off selection, showed markedly limited and distinct ranges of significantly favorable TMB cut-offs. Equivalent results were obtained in the analyses of pooled tumors.
Conclusions: Our finding-the correlation that TMB-H is more robustly associated with favorable ICB treatment outcomes in cancer types with higher TMBs-can be used to predict whether TMB could be a robust predictive biomarker in cancer types for which TMB data are available, but ICB treatment has not been investigated. This theory was tested in cancer of unknown primary successfully. Additionally, the universal TMB cut-off of 13 mut/Mb might reveal a general requirement to trigger the sequential cascade from somatic mutations to an effective antitumor immunity.
Keywords: immunotherapy.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
