Tumor mutational burden predicts the efficacy of pembrolizumab monotherapy: a pan-tumor retrospective analysis of participants with advanced solid tumors
- PMID: 35101941
- PMCID: PMC8804694
- DOI: 10.1136/jitc-2021-003091
Tumor mutational burden predicts the efficacy of pembrolizumab monotherapy: a pan-tumor retrospective analysis of participants with advanced solid tumors
Abstract
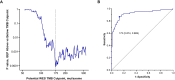
Background: Several studies have evaluated the relationship between tumor mutational burden (TMB) and outcomes of immune checkpoint inhibitors. In the phase II KEYNOTE-158 study of pembrolizumab monotherapy for previously treated recurrent or metastatic cancer, high TMB as assessed by the FoundationOne CDx was associated with an improved objective response rate (ORR).
Methods: We retrospectively assessed the relationship between TMB and efficacy in participants with previously treated advanced solid tumors enrolled in 12 trials that evaluated pembrolizumab monotherapy, including 3 randomized trials that compared pembrolizumab with chemotherapy. TMB was assessed in formalin-fixed, paraffin-embedded pretreatment tumor samples by whole-exome sequencing. High TMB was defined as ≥175 mutations/exome. Microsatellite instability (MSI) phenotype was based on whole-exome sequencing results. Programmed death ligand 1 (PD-L1) expression was assessed by immunohistochemistry. The primary end point was ORR assessed per RECIST V.1.1 by independent central review. Other end points included progression-free survival (PFS) assessed per RECIST V.1.1 by independent central review and overall survival (OS).
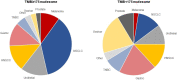
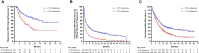
Results: Of the 2234 participants in the analysis, 1772 received pembrolizumab monotherapy and 462 received chemotherapy. Among the pembrolizumab-treated participants, ORR was 31.4% (95% CI 27.1 to 36.0) in the 433 participants with TMB ≥175 mutations/exome and 9.5% (95% CI 8.0 to 11.2) in the 1339 participants with TMB <175 mutations/exome. The association of TMB with ORR was observed regardless of PD-L1 expression and not driven by specific tumor types or participants with very high TMB or high MSI. In the 3 randomized controlled trials, TMB was associated with ORR (p≤0.016), PFS (p≤0.005), and OS (p≤0.029) of pembrolizumab but not of chemotherapy (p≥0.340, p≥0.643, and p≥0.174, respectively), and pembrolizumab improved efficacy versus chemotherapy in participants with TMB ≥175 mutations/exome.
Conclusions: TMB ≥175 mutations/exome is associated with clinically meaningful improvement in the efficacy of pembrolizumab monotherapy and improved outcomes for pembrolizumab versus chemotherapy across a wide range of previously treated advanced solid tumor types. These data suggest TMB has broad clinical utility irrespective of tumor type, PD-L1 expression, or MSI status and support its use as a predictive biomarker for pembrolizumab monotherapy in participants with previously treated advanced solid tumors.
Trial registration: ClinicalTrials.gov NCT01295827 NCT01704287 NCT01905657 NCT01848834 NCT02054806 NCT02256436 NCT02255097 NCT02335411 NCT02370498 NCT02447003 NCT02674061 NCT02787005.
Keywords: immunotherapy; programmed cell death 1 receptor; tumor biomarkers.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: RC, DA-G, AA, LX, AL, LL, FJ, EHR, and JL are full-time employees of Merck Sharp & Dohme Corp, a subsidiary of Merck & Co, Inc, Kenilworth, New Jersey, USA and hold stock in Merck & Co, Inc, Kenilworth, New Jersey, USA. XQL is a full-time employee of MSD China. AS was a full-time employee of Merck Sharp & Dohme Corp, a subsidiary of Merck & Co, Inc, Kenilworth, New Jersey, USA at the time the study was conducted.
Figures


References
-
- Marabelle A, Fakih M, Lopez J, et al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol 2020;21:1353–65. 10.1016/S1470-2045(20)30445-9 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
