Sequence-specific RNA recognition by an RGG motif connects U1 and U2 snRNP for spliceosome assembly
- PMID: 35101980
- PMCID: PMC8833184
- DOI: 10.1073/pnas.2114092119
Sequence-specific RNA recognition by an RGG motif connects U1 and U2 snRNP for spliceosome assembly
Abstract
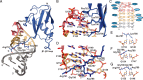
In mammals, the structural basis for the interaction between U1 and U2 small nuclear ribonucleoproteins (snRNPs) during the early steps of splicing is still elusive. The binding of the ubiquitin-like (UBL) domain of SF3A1 to the stem-loop 4 of U1 snRNP (U1-SL4) contributes to this interaction. Here, we determined the 3D structure of the complex between the UBL of SF3A1 and U1-SL4 RNA. Our crystallography, NMR spectroscopy, and cross-linking mass spectrometry data show that SF3A1-UBL recognizes, sequence specifically, the GCG/CGC RNA stem and the apical UUCG tetraloop of U1-SL4. In vitro and in vivo mutational analyses support the observed intermolecular contacts and demonstrate that the carboxyl-terminal arginine-glycine-glycine-arginine (RGGR) motif of SF3A1-UBL binds sequence specifically by inserting into the RNA major groove. Thus, the characterization of the SF3A1-UBL/U1-SL4 complex expands the repertoire of RNA binding domains and reveals the capacity of RGG/RG motifs to bind RNA in a sequence-specific manner.
Keywords: RGG motif; spliceosome assembly; splicing; ubiquitin-like domain.
Copyright © 2022 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no competing interest.
Figures




Similar articles
-
Identification of a noncanonical RNA binding domain in the U2 snRNP protein SF3A1.RNA. 2019 Nov;25(11):1509-1521. doi: 10.1261/rna.072256.119. Epub 2019 Aug 5. RNA. 2019. PMID: 31383795 Free PMC article.
-
Structural insights into recognition of SL4, the UUCG stem-loop, of human U1 snRNA by the ubiquitin-like domain, including the C-terminal tail in the SF3A1 subunit of U2 snRNP.J Biochem. 2023 Jul 31;174(2):203-216. doi: 10.1093/jb/mvad033. J Biochem. 2023. PMID: 37094335
-
Stem-loop 4 of U1 snRNA is essential for splicing and interacts with the U2 snRNP-specific SF3A1 protein during spliceosome assembly.Genes Dev. 2014 Nov 15;28(22):2518-31. doi: 10.1101/gad.248625.114. Genes Dev. 2014. PMID: 25403181 Free PMC article.
-
U1 snRNP Biogenesis Defects in Neurodegenerative Diseases.Chembiochem. 2024 May 2;25(9):e202300864. doi: 10.1002/cbic.202300864. Epub 2024 Mar 25. Chembiochem. 2024. PMID: 38459794 Review.
-
SF3a1: A Novel Potential Tumor Biomarker or Therapeutic Target.J Cancer. 2025 Apr 9;16(7):2353-2359. doi: 10.7150/jca.103209. eCollection 2025. J Cancer. 2025. PMID: 40302801 Free PMC article. Review.
Cited by
-
Structural insights into human exon-defined spliceosome prior to activation.Cell Res. 2024 Jun;34(6):428-439. doi: 10.1038/s41422-024-00949-w. Epub 2024 Apr 24. Cell Res. 2024. PMID: 38658629 Free PMC article.
-
Examining the capacity of human U1 snRNA variants to facilitate pre-mRNA splicing.RNA. 2024 Feb 16;30(3):271-280. doi: 10.1261/rna.079892.123. RNA. 2024. PMID: 38164604 Free PMC article.
-
Biomolecular condensates-Prerequisites for anhydrobiosis?Protein Sci. 2025 Jul;34(7):e70192. doi: 10.1002/pro.70192. Protein Sci. 2025. PMID: 40521613 Review.
-
Major-groove sequence-specific RNA recognition by LoaP, a paralog of transcription elongation factor NusG.Structure. 2024 Sep 5;32(9):1488-1497.e5. doi: 10.1016/j.str.2024.06.001. Epub 2024 Jul 2. Structure. 2024. PMID: 38959899 Free PMC article.
-
Intramolecular autoinhibition regulates the selectivity of PRPF40A tandem WW domains for proline-rich motifs.Nat Commun. 2024 May 8;15(1):3888. doi: 10.1038/s41467-024-48004-x. Nat Commun. 2024. PMID: 38719828 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

