Robust induction of B cell and T cell responses by a third dose of inactivated SARS-CoV-2 vaccine
- PMID: 35102140
- PMCID: PMC8803973
- DOI: 10.1038/s41421-022-00373-7
Robust induction of B cell and T cell responses by a third dose of inactivated SARS-CoV-2 vaccine
Abstract
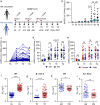
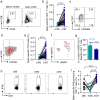
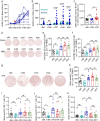
SARS-CoV-2 inactivated vaccines have shown remarkable efficacy in clinical trials, especially in reducing severe illness and casualty. However, the waning of humoral immunity over time has raised concern over the durability of immune memory following vaccination. Thus, we conducted a nonrandomized trial among the healthcare workers (HCWs) to investigate the long-term sustainability of SARS-CoV-2-specific B cells and T cells stimulated by inactivated vaccines and the potential need for a third booster dose. Although neutralizing antibodies elicited by the standard two-dose vaccination schedule dropped from a peak of 29.3 arbitrary units (AU)/mL to 8.8 AU/mL 5 months after the second vaccination, spike-specific memory B and T cells were still detectable, forming the basis for a quick recall response. As expected, the faded humoral immune response was vigorously elevated to 63.6 AU/mL by 7.2 folds 1 week after the third dose along with abundant spike-specific circulating follicular helper T cells in parallel. Meanwhile, spike-specific CD4+ and CD8+ T cells were also robustly elevated by 5.9 and 2.7 folds respectively. Robust expansion of memory pools by the third dose potentiated greater durability of protective immune responses. Another key finding in this trial was that HCWs with low serological response to two doses were not truly "non-responders" but fully equipped with immune memory that could be quickly recalled by a third dose even 5 months after the second vaccination. Collectively, these data provide insights into the generation of long-term immunological memory by the inactivated vaccine, which could be rapidly recalled and further boosted by a third dose.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Crook H, Raza S, Nowell J, Young M, Edison P. Long covid-mechanisms, risk factors, and management. BMJ. 2021;374:n1648. - PubMed
-
- COVID-19 Map – Johns Hopkins Coronavirus Resource Center. https://coronavirus.jhu.edu/map.html (2021).
-
- Krause, P. R. & Gruber, M. F. Emergency use authorization of covid vaccines – Safety and efficacy follow-up considerations. N. Engl. J. Med.383, e107 (2020). - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

