A simple additive staging system for newly diagnosed multiple myeloma
- PMID: 35102148
- PMCID: PMC8803917
- DOI: 10.1038/s41408-022-00611-x
A simple additive staging system for newly diagnosed multiple myeloma
Abstract
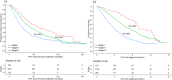
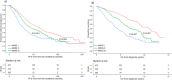
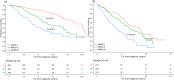
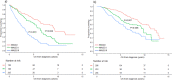
Risk stratification in multiple myeloma is important for prognostication, patient selection for clinical trials, and comparison of treatment approaches. We developed and validated a staging system that incorporates additional FISH abnormalities not included in the R-ISS and reflects the additive effects of co-occurring high-risk disease features. We first evaluated the prognostic value of predefined cytogenetic and laboratory abnormalities in 2556 Mayo Clinic patients diagnosed between February 2004 and June 2019. We then used data from 1327 patients to develop a risk stratification model and validated this in 502 patients enrolled in the MMRF CoMMpass study. On multivariate analysis, high-risk IgH translocations [risk ratio (RR): 1.7], 1q gain/amplification (RR: 1.4), chromosome17 abnormalities (RR: 1.6), ISS III (RR: 1.7), and elevated LDH (RR: 1.3) were independently associated with decreased overall survival (OS). Among 1327 evaluable patients, OS was 11.0 (95% CI: 9.2-12.6), 7.0 (95% CI: 6.3-9.2), and 4.5 (95% CI: 3.7-5.2) years in patients with 0 (stage I), 1 (stage II), and ≥2 (stage III) high-risk factors, respectively. In the MMRF cohort, median OS was 7.8 (95% CI: NR-NR), 6.0 (95% CI: 5.7-NR), and 4.3 (95% CI: 2.7-NR) years in the 3 groups, respectively (P < 0.001). This 5-factor, 3-tier system is easy to implement in practice and improves upon the current R-ISS.
© 2022. The Author(s).
Conflict of interest statement
PK received research funding from Takeda Pharmaceuticals, Celgene, and Amgen. AD received research funding from Celgene, Millennium Pharmaceuticals, Pfizer, and Janssen and received a travel grant from Pfizer. MAG served as a consultant for Millennium Pharmaceuticals and received honoraria from Celgene, Millennium Pharmaceuticals, Onyx Pharmaceuticals, Novartis, GlaxoSmithKline, Prothena, Ionis Pharmaceuticals, and Amgen. MQL received research funding from Celgene. NL serves on an advisory board for Takeda Pharmaceuticals. RF served as a consultant for Amgen, BMS, Celgene, Takeda, Bayer, Janssen, Novartis, Pharmacyclics, Sanofi, Karyopharm, Merck, Juno, Kite, Aduro, OncoTracker, Oncopeptides, GSK, and AbbVie, and is on the scientific Advisory Board for Adaptive Biotechnologies, Caris Life Sciences and OncoTracker. SKK served as a consultant for Celgene, Millennium Pharmaceuticals, Onyx Pharmaceuticals, Janssen, and Bristol-Myers Squibb and received research funding from Celgene, Millennium Pharmaceuticals, Novartis, Onyx Pharmaceuticals, AbbVie, Janssen, and Bristol-Myers Squibb. The remaining authors declare no competing financial interests.
Figures


References
-
- Durie BGM, Hoering A, Sexton R, Abidi MH, Epstein J, Rajkumar SV, et al. Longer term follow-up of the randomized phase III trial SWOG S0777: bortezomib, lenalidomide and dexamethasone vs. lenalidomide and dexamethasone in patients (Pts) with previously untreated multiple myeloma without an intent for immediate autologous stem cell transplant (ASCT) Blood Cancer J. 2020;10:53. doi: 10.1038/s41408-020-0311-8. - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

