Mobilisation of deep crustal sulfide melts as a first order control on upper lithospheric metallogeny
- PMID: 35102157
- PMCID: PMC8803977
- DOI: 10.1038/s41467-022-28275-y
Mobilisation of deep crustal sulfide melts as a first order control on upper lithospheric metallogeny
Abstract
Magmatic arcs are terrestrial environments where lithospheric cycling and recycling of metals and volatiles is enhanced. However, the first-order mechanism permitting the episodic fluxing of these elements from the mantle through to the outer Earth's spheres has been elusive. To address this knowledge gap, we focus on the textural and minero-chemical characteristics of metal-rich magmatic sulfides hosted in amphibole-olivine-pyroxene cumulates in the lowermost crust. We show that in cumulates that were subject to increasing temperature due to prolonged mafic magmatism, which only occurs episodically during the complex evolution of any magmatic arc, Cu-Au-rich sulfide can exist as liquid while Ni-Fe rich sulfide occurs as a solid phase. This scenario occurs within a 'Goldilocks' temperature zone at ~1100-1200 °C, typical of the base of the crust in arcs, which permits episodic fractionation and mobilisation of Cu-Au-rich sulfide liquid into permeable melt networks that may ascend through the lithosphere providing metals for porphyry and epithermal ore deposits.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




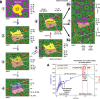
References
-
- Richards JP. Cumulative factors in the generation of giant calc-alkaline porphyry Cu deposits. Super. Porphyry Copp. Gold. Depos. Glob. Perspect. 2005;1:7–25.
-
- Hawkesworth CJ, Kemp AIS. Evolution of the continental crust. Nature. 2006;443:811–817. - PubMed
-
- Richards JP. Magmatic to hydrothermal metal fluxes in convergent and collided margins. Ore Geol. Rev. 2011;40:1–26.
-
- Sillitoe RH. Porphyry copper systems. Econ. Geol. 2010;105:3–41.
-
- Wilkinson JJ. Triggers for the formation of porphyry ore deposits in magmatic arcs. Nat. Geosci. 2013;6:917–925.
Grants and funding
- NE/P017053/1/RCUK | Natural Environment Research Council (NERC)
- NE/P017312/1/RCUK | Natural Environment Research Council (NERC)
- CE11E0070/Department of Education and Training | ARC | Centre of Excellence for Core to Crust Fluid Systems, Australian Research Council (ARC Centre of Excellence for Core to Crust Fluid Systems)
LinkOut - more resources
Full Text Sources
Miscellaneous

