Evaluation of the EMPAR study population on the basis of metabolic phenotypes of selected pharmacogenes
- PMID: 35102241
- PMCID: PMC8975744
- DOI: 10.1038/s41397-022-00268-6
Evaluation of the EMPAR study population on the basis of metabolic phenotypes of selected pharmacogenes
Abstract
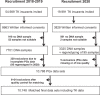
The impact of genetic variability of pharmacogenes as a possible risk factor for adverse drug reactions is elucidated in the EMPAR (Einfluss metabolischer Profile auf die Arzneimitteltherapiesicherheit in der Routineversorgung/English: influence of metabolic profiles on the safety of drug therapy in routine care) study. EMPAR evaluates possible associations of pharmacogenetically predicted metabolic profiles relevant for the metabolism of frequently prescribed cardiovascular drugs. Based on a German study population of 10,748 participants providing access to healthcare claims data and DNA samples for pharmacogenetic assessment, first analyses were performed and evaluated. The aim of this first evaluation was the characterization of the study population with regard to general parameters such as age, gender, comorbidity, and polypharmacy at baseline (baseline year) as well as important combinations of cardiovascular drugs with relevant genetic variants and predicted metabolic phenotypes. The study was registered in the German Clinical Trials Register (DRKS) on July 6, 2018 (DRKS00013909).
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Leendertse AJ, Egberts AC, Stoker LJ, van den Bemt PM. Frequency of and risk factors for preventable medication-related hospital admissions in the Netherlands. Arch Intern Med. 2008;168:1890–6. - PubMed
-
- Ji Y, Skierka JM, Blommel JH, Moore BE, VanCuyk DL, Bruflat JK, et al. Preemptive pharmacogenomic testing for precision medicine: a comprehensive analysis of five actionable pharmacogenomic genes using next-generation DNA sequencing and a customized CYP2D6 genotyping cascade. J Mol Diagn. 2016;18:438–45. doi: 10.1016/j.jmoldx.2016.01.003. - DOI - PMC - PubMed
-
- Unternehmensdaten | Die Techniker – Presse und Politik. 2021 https://www.tk.de/presse/tk-unternehmensdaten-2051018. Accessed 20 July 2020.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

