Targeting mAKAPβ expression as a therapeutic approach for ischemic cardiomyopathy
- PMID: 35102273
- PMCID: PMC9339585
- DOI: 10.1038/s41434-022-00321-w
Targeting mAKAPβ expression as a therapeutic approach for ischemic cardiomyopathy
Abstract
Ischemic cardiomyopathy is a leading cause of death and an unmet clinical need. Adeno-associated virus (AAV) gene-based therapies hold great promise for treating and preventing heart failure. Previously we showed that muscle A-kinase Anchoring Protein β (mAKAPβ, AKAP6β), a scaffold protein that organizes perinuclear signalosomes in the cardiomyocyte, is a critical regulator of pathological cardiac hypertrophy. Here, we show that inhibition of mAKAPβ expression in stressed adult cardiomyocytes in vitro was cardioprotective, while conditional cardiomyocyte-specific mAKAP gene deletion in mice prevented pathological cardiac remodeling due to myocardial infarction. We developed a new self-complementary serotype 9 AAV gene therapy vector expressing a short hairpin RNA for mAKAPβ under the control of a cardiomyocyte-specific promoter (AAV9sc.shmAKAP). This vector efficiently downregulated mAKAPβ expression in the mouse heart in vivo. Expression of the shRNA also inhibited mAKAPβ expression in human induced cardiomyocytes in vitro. Following myocardial infarction, systemic administration of AAV9sc.shmAKAP prevented the development of pathological cardiac remodeling and heart failure, providing long-term restoration of left ventricular ejection fraction. Our findings provide proof-of-concept for mAKAPβ as a therapeutic target for ischemic cardiomyopathy and support the development of a translational pipeline for AAV9sc.shmAKAP for the treatment of heart failure.
© 2022. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
Competing Interests
Drs. Kapiloff and Li are inventors of patent-protected intellectual property concerning the targeting of mAKAPβ signalosomes to treat heart failure, by which they, the University of Miami, and Stanford University may gain royalties from future commercialization. Dr. Kapiloff holds equity in Anchored RSK3 Inhibitors, LLC, and Cardiac RSK3 Inhibitors, LLC, companies interested in developing mAKAP signalosome-targeted therapies.
Figures

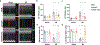


References
-
- Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, Callaway CW, et al. Heart Disease and Stroke Statistics-2021 Update: A Report From the American Heart Association. Circulation 2021;143(8):e254–e743. - PubMed
-
- McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Bohm M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2021;42(36):3599–726. - PubMed
-
- Nakamura M, Sadoshima J. Mechanisms of physiological and pathological cardiac hypertrophy. Nature reviews Cardiology 2018;15(7):387–407. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

