Quantifying Cell-Derived Changes in Collagen Synthesis, Alignment, and Mechanics in a 3D Connective Tissue Model
- PMID: 35102708
- PMCID: PMC8981917
- DOI: 10.1002/advs.202103939
Quantifying Cell-Derived Changes in Collagen Synthesis, Alignment, and Mechanics in a 3D Connective Tissue Model
Abstract
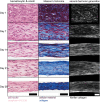
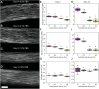
Dysregulation of extracellular matrix (ECM) synthesis, organization, and mechanics are hallmark features of diseases like fibrosis and cancer. However, most in vitro models fail to recapitulate the three-dimensional (3D) multi-scale hierarchical architecture of collagen-rich tissues and as a result, are unable to mirror native or disease phenotypes. Herein, using primary human fibroblasts seeded into custom fabricated 3D non-adhesive agarose molds, a novel strategy is proposed to direct the morphogenesis of engineered 3D ring-shaped tissue constructs with tensile and histological properties that recapitulate key features of fibrous connective tissue. To characterize the shift from monodispersed cells to a highly-aligned, collagen-rich matrix, a multi-modal approach integrating histology, multiphoton second-harmonic generation, and electron microscopy is employed. Structural changes in collagen synthesis and alignment are then mapped to functional differences in tissue mechanics and total collagen content. Due to the absence of an exogenously added scaffolding material, this model enables the direct quantification of cell-derived changes in 3D matrix synthesis, alignment, and mechanics in response to the addition or removal of relevant biomolecular perturbations. To illustrate this, the effects of nutrient composition, fetal bovine serum, rho-kinase inhibitor, and pro- and anti-fibrotic compounds on ECM synthesis, 3D collagen architecture, and mechanophenotype are quantified.
Keywords: 3D tissue engineering; TGF-β1; collagen; connective tissue; extracellular matrix; fibroblast; fibrosis; mechanics; mechanophenotype.
© 2022 The Authors. Advanced Science published by Wiley-VCH GmbH.
Conflict of interest statement
J.R.M. has an equity interest in Microtissues, Inc. This relationship has been reviewed and is managed by Brown University in accordance with its conflict of interest policies.
Figures





Similar articles
-
Directing fibroblast self-assembly to fabricate highly-aligned, collagen-rich matrices.Acta Biomater. 2018 Nov;81:70-79. doi: 10.1016/j.actbio.2018.09.030. Epub 2018 Sep 27. Acta Biomater. 2018. PMID: 30267883
-
Geometrical confinement controls cell, ECM and vascular network alignment during the morphogenesis of 3D bioengineered human connective tissues.Acta Biomater. 2021 Sep 1;131:341-354. doi: 10.1016/j.actbio.2021.06.022. Epub 2021 Jun 16. Acta Biomater. 2021. PMID: 34144214
-
In the beginning there were soft collagen-cell gels: towards better 3D connective tissue models?Exp Cell Res. 2013 Oct 1;319(16):2460-9. doi: 10.1016/j.yexcr.2013.07.001. Epub 2013 Jul 12. Exp Cell Res. 2013. PMID: 23856376 Review.
-
Micrometer scale guidance of mesenchymal stem cells to form structurally oriented large-scale tissue engineered cartilage.Acta Biomater. 2017 Sep 15;60:210-219. doi: 10.1016/j.actbio.2017.07.016. Epub 2017 Jul 11. Acta Biomater. 2017. PMID: 28709984 Free PMC article.
-
Fibroblast mechanics in 3D collagen matrices.Adv Drug Deliv Rev. 2007 Nov 10;59(13):1299-305. doi: 10.1016/j.addr.2007.08.006. Epub 2007 Aug 14. Adv Drug Deliv Rev. 2007. PMID: 17825456 Free PMC article. Review.
Cited by
-
Exogenous ECM in an environmentally-mediated in vitro model for cardiac fibrosis.bioRxiv [Preprint]. 2024 Aug 21:2024.08.20.608840. doi: 10.1101/2024.08.20.608840. bioRxiv. 2024. PMID: 39229021 Free PMC article. Preprint.
-
Biochemical composition of the glomerular extracellular matrix in patients with diabetic kidney disease.World J Diabetes. 2022 Jul 15;13(7):498-520. doi: 10.4239/wjd.v13.i7.498. World J Diabetes. 2022. PMID: 36051430 Free PMC article. Review.
-
Mueller matrix analysis of a biologically sourced engineered tissue construct as polarimetric phantom.J Biomed Opt. 2024 Oct;29(10):106002. doi: 10.1117/1.JBO.29.10.106002. Epub 2024 Oct 29. J Biomed Opt. 2024. PMID: 39474362 Free PMC article.
-
An in vitro model to measure the strength and stiffness of the extracellular matrix synthesized de novo by human fibroblasts.In Vitro Model. 2025 Mar 7;4(1):59-69. doi: 10.1007/s44164-025-00081-y. eCollection 2025 Feb. In Vitro Model. 2025. PMID: 40160211
-
Lab-grown, 3D extracellular matrix particles improve cardiac function and morphology in myocardial ischemia.Am J Physiol Heart Circ Physiol. 2025 Feb 1;328(2):H221-H234. doi: 10.1152/ajpheart.00581.2024. Epub 2024 Dec 20. Am J Physiol Heart Circ Physiol. 2025. PMID: 39705507 Free PMC article.
References
-
- Goh K. L., Holmes D. F., Lu H.‐Y., Richardson S., Kadler K. E., Purslow P. P., Wess T. J., J. Biomech. Eng. 2008, 130, 2. - PubMed
-
- Vanakker O., Callewaert B., Malfait F., Coucke P., Annual Review of Genomics and Human Genetics 2015, 16, 229. - PubMed
-
- Tilstra D. J., Byers P. H., Annu. Rev. Med. 1994, 45, 149. - PubMed
-
- Roca‐Cusachs P., Conte V., Trepat X., Nat. Cell Biol. 2017, 19, 742. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
