Ibrutinib plus Obinutuzumab as Frontline Therapy for Chronic Lymphocytic Leukemia Is Associated with a Lower Rate of Infusion-Related Reactions and with Sustained Remissions after Ibrutinib Discontinuation: A Single-Arm, Open-Label, Phase 1b/2 Clinical Trial NCT0231576
- PMID: 35103064
- PMCID: PMC8800600
- DOI: 10.1155/2022/4450824
Ibrutinib plus Obinutuzumab as Frontline Therapy for Chronic Lymphocytic Leukemia Is Associated with a Lower Rate of Infusion-Related Reactions and with Sustained Remissions after Ibrutinib Discontinuation: A Single-Arm, Open-Label, Phase 1b/2 Clinical Trial NCT0231576
Abstract
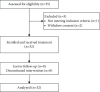
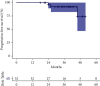
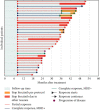
Ibrutinib-based therapies are costly and require continuous administration. We hypothesized combining BTK inhibition with anti-CD20 monoclonal antibodies would yield deep remissions allowing discontinuation. We enrolled 32 therapy-naïve CLL patients to receive ibrutinib plus obinutuzumab, followed by single-agent ibrutinib. Patients could discontinue ibrutinib after 36 months with sustained complete response (CR). We evaluated treatment safety, efficacy, and outcomes after ibrutinib discontinuation. The overall response rate was 100%, 28% achieved a CR, and 12.5% achieved bone marrow undetectable minimal residual disease. At a three-year median follow-up, 91% remain in remission with 100% overall survival. Five patients in sustained CR stopped ibrutinib and have not progressed. Eight non-CR patients discontinued for other reasons, with only two progressing. The treatment was safe, with a lower IRR rate. All patients responded to treatment with longer time-to-progression after discontinuation of ibrutinib. Our data support the evaluation of ibrutinib discontinuation strategies in more extensive clinical trials (https://Clinicaltrials.gov Identifier https://clinicaltrials.gov/ct2/show/NCT02315768).
Copyright © 2022 Januario E. Castro et al.
Conflict of interest statement
JVL, PALD, EFMC, JVF, JEGR, CJ, CM, and AH disclose no conflicts of interest. MYC is a consultant at AbbVie, Speakers Bureau. TJK is a consultant at Pharmacyclics, Honoraria, Membership on an entity's Board of Directors or advisory committees and provided research funding. JEC is a consultant at Pharmacyclics, LLC, an AbbVie company. CAC has equity ownership and employment at AbbVie. For other authors, research was performed while employed as an investigator of this study at UCSD.
Figures

References
Associated data
LinkOut - more resources
Full Text Sources
Medical

