Adapting Serosurveys for the SARS-CoV-2 Vaccine Era
- PMID: 35103246
- PMCID: PMC8755308
- DOI: 10.1093/ofid/ofab632
Adapting Serosurveys for the SARS-CoV-2 Vaccine Era
Abstract
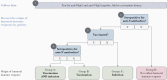
Population-level immune surveillance, which includes monitoring exposure and assessing vaccine-induced immunity, is a crucial component of public health decision-making during a pandemic. Serosurveys estimating the prevalence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antibodies in the population played a key role in characterizing SARS-CoV-2 epidemiology during the early phases of the pandemic. Existing serosurveys provide infrastructure to continue immune surveillance but must be adapted to remain relevant in the SARS-CoV-2 vaccine era. Here, we delineate how SARS-CoV-2 serosurveys should be designed to distinguish infection- and vaccine-induced humoral immune responses to efficiently monitor the evolution of the pandemic. We discuss how serosurvey results can inform vaccine distribution to improve allocation efficiency in countries with scarce vaccine supplies and help assess the need for booster doses in countries with substantial vaccine coverage.
Keywords: 19; 2; COVID; CoV; SARS; cross; sectional; seroprevalence; vaccines.
© The Author(s) 2021. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Figures
References
-
- Planas D, Veyer D, Baidaliuk A, et al. . Reduced sensitivity of SARS-CoV-2 variant Delta to antibody neutralization. Nature 2021; 596:276–80. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

