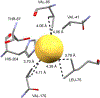
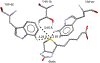
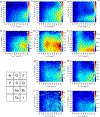
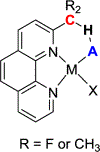
C-H⋯S hydrogen bonding interactions
- PMID: 35103265
- PMCID: PMC9088610
- DOI: 10.1039/d1cs00838b
C-H⋯S hydrogen bonding interactions
Abstract
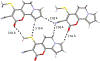
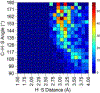
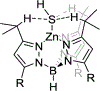
The short C-H⋯S contacts found in available structural data for both small molecules and larger biomolecular systems suggest that such contacts are an often overlooked yet important stabilizing interaction. Moreover, many of these short C-H⋯S contacts meet the definition of a hydrogen bonding interaction. Using available structural data from the Cambridge Structural Database (CSD), as well as selected examples from the literature in which important C-H⋯S contacts may have been overlooked, we highlight the generality of C-H⋯S hydrogen bonding as an important stabilizing interaction. To uncover and establish the generality of these interactions, we compare C-H⋯S contacts with other traditional hydrogen bond donors and acceptors as well as investigate how coordination number and metal bonding affect the preferred geometry of interactions in the solid state. This work establishes that the C-H⋯S bond meets the definition of a hydrogen bond and serves as a guide to identify C-H⋯S hydrogen bonds in diverse systems.
Conflict of interest statement
Conflicts of interest
There are no conflicts to declare.
Figures

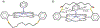
Similar articles
-
The C-H···S-S hydrogen bonding in diethyl disulfide⋯difluoromethane: a combined microwave spectroscopic and computational study.Phys Chem Chem Phys. 2024 Dec 11;26(48):29940-29947. doi: 10.1039/d4cp03994g. Phys Chem Chem Phys. 2024. PMID: 39618337
-
Unveiling the underappreciated: The bonding features of C-H⋯S-S interactions observed from rotational spectroscopy.J Chem Phys. 2024 Apr 7;160(13):134302. doi: 10.1063/5.0200788. J Chem Phys. 2024. PMID: 38557843
-
Conformation control through concurrent N-H⋯S and N-H⋯O[double bond, length as m-dash]C hydrogen bonding and hyperconjugation effects.Chem Sci. 2020 Aug 11;11(34):9191-9197. doi: 10.1039/d0sc03339a. Chem Sci. 2020. PMID: 34123167 Free PMC article.
-
(E)-2-[1-(1-Benzothio-phen-3-yl)ethyl-idene]hydrazinecarbothio-amide methanol hemisolvate.Acta Crystallogr Sect E Struct Rep Online. 2008 May 10;64(Pt 6):o1022-3. doi: 10.1107/S160053680801297X. Acta Crystallogr Sect E Struct Rep Online. 2008. PMID: 21202547 Free PMC article.
-
Hydrogen bond types which do not fit accepted definitions.Chem Commun (Camb). 2024 Jun 13;60(49):6239-6255. doi: 10.1039/d4cc01769b. Chem Commun (Camb). 2024. PMID: 38828514 Review.
Cited by
-
Columnar Mesomorphism in a Methylthio-Decorated Triindole for Enhanced Charge Transport.ACS Appl Electron Mater. 2024 Jun 11;6(6):4709-4717. doi: 10.1021/acsaelm.4c00693. eCollection 2024 Jun 25. ACS Appl Electron Mater. 2024. PMID: 38947954 Free PMC article.
-
Synthesis and reactivity of a tris(carbene) zinc chloride complex.Dalton Trans. 2022 Oct 4;51(38):14563-14567. doi: 10.1039/d2dt02809c. Dalton Trans. 2022. PMID: 36074723 Free PMC article.
-
Absolute structure determination of Berkecoumarin by X-ray and electron diffraction.Acta Crystallogr C Struct Chem. 2024 May 1;80(Pt 5):143-147. doi: 10.1107/S2053229624003061. Epub 2024 Apr 10. Acta Crystallogr C Struct Chem. 2024. PMID: 38598330 Free PMC article.
-
Novel enantiopure δ-thiolactones: synthesis, structural characterization, and reactivity studies.RSC Adv. 2024 Dec 24;14(54):40287-40298. doi: 10.1039/d4ra07780f. eCollection 2024 Dec 17. RSC Adv. 2024. PMID: 39720259 Free PMC article.
-
Unravelling the potency of the 4-oxo-2-thioxo-1,2,3,4-tetrahydropyrimidine-5-carbonitrile scaffold with S-arylamide hybrids as PIM-1 kinase inhibitors: synthesis, biological activity and in silico studies.RSC Med Chem. 2025 Mar 28. doi: 10.1039/d5md00021a. Online ahead of print. RSC Med Chem. 2025. PMID: 40162200 Free PMC article.
References
-
- Pauling LC, The Nature of the Chemical Bond, Cornell University Press, Ithaca, NY, 1st ed.,1939; 2nd ed., 1940, 3rd ed., 1960.
-
- Liptrot DJ and Power PP, Nature Reviews Chemistry, 2017, 1, 0004.
-
- Meyer EA, Castellano RK and Diederich F, Angew. Chem. Int. Ed, 2003, 42, 1210–1250. - PubMed
-
- Paulini R, Müller K and Diederich F, Angew. Chem. Int. Ed, 2005, 44, 1788–1805. - PubMed
-
- Beno BR, Yeung K-S, Bartberger MD, Pennington LD and Meanwell NA, J. Med. Chem, 2015, 58, 4383–4438. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

