C-H⋯S hydrogen bonding interactions
- PMID: 35103265
- PMCID: PMC9088610
- DOI: 10.1039/d1cs00838b
C-H⋯S hydrogen bonding interactions
Abstract
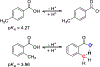
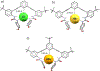
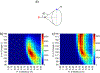
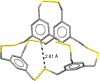
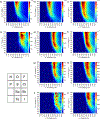
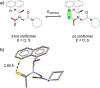
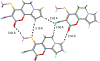
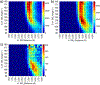
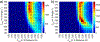
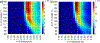
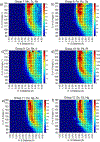
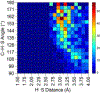
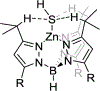
The short C-H⋯S contacts found in available structural data for both small molecules and larger biomolecular systems suggest that such contacts are an often overlooked yet important stabilizing interaction. Moreover, many of these short C-H⋯S contacts meet the definition of a hydrogen bonding interaction. Using available structural data from the Cambridge Structural Database (CSD), as well as selected examples from the literature in which important C-H⋯S contacts may have been overlooked, we highlight the generality of C-H⋯S hydrogen bonding as an important stabilizing interaction. To uncover and establish the generality of these interactions, we compare C-H⋯S contacts with other traditional hydrogen bond donors and acceptors as well as investigate how coordination number and metal bonding affect the preferred geometry of interactions in the solid state. This work establishes that the C-H⋯S bond meets the definition of a hydrogen bond and serves as a guide to identify C-H⋯S hydrogen bonds in diverse systems.
Conflict of interest statement
Conflicts of interest
There are no conflicts to declare.
Figures
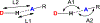
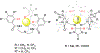

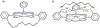
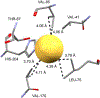
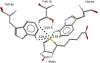
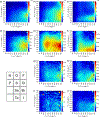
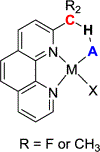
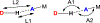
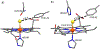
References
-
- Pauling LC, The Nature of the Chemical Bond, Cornell University Press, Ithaca, NY, 1st ed.,1939; 2nd ed., 1940, 3rd ed., 1960.
-
- Liptrot DJ and Power PP, Nature Reviews Chemistry, 2017, 1, 0004.
-
- Meyer EA, Castellano RK and Diederich F, Angew. Chem. Int. Ed, 2003, 42, 1210–1250. - PubMed
-
- Paulini R, Müller K and Diederich F, Angew. Chem. Int. Ed, 2005, 44, 1788–1805. - PubMed
-
- Beno BR, Yeung K-S, Bartberger MD, Pennington LD and Meanwell NA, J. Med. Chem, 2015, 58, 4383–4438. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

