Enantioselective Recognition of Helicenes by a Tailored Chiral Benzo[ghi]perylene Trisimide π-Scaffold
- PMID: 35103371
- PMCID: PMC9303377
- DOI: 10.1002/anie.202117625
Enantioselective Recognition of Helicenes by a Tailored Chiral Benzo[ghi]perylene Trisimide π-Scaffold
Abstract
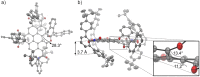
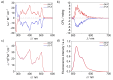
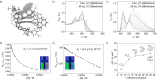
Enantioselective molecular recognition of chiral molecules that lack specific interaction sites for hydrogen bonding or Lewis acid-base interactions remains challenging. Here we introduce the concept of tailored chiral π-surfaces toward the maximization of shape complementarity. As we demonstrate for helicenes it is indeed possible by pure van-der-Waals interactions (π-π interactions and CH-π interactions) to accomplish enantioselective binding. This is shown for a novel benzo[ghi]perylene trisimide (BPTI) receptor whose π-scaffold is contorted into a chiral plane by functionalization with 1,1'-bi-2-naphthol (BINOL). Complexation experiments of enantiopure (P)-BPTI with (P)- and (M)-[6]helicene afforded binding constants of 10 700 M-1 and 550 M-1 , respectively, thereby demonstrating the pronounced enantiodifferentiation by the homochiral π-scaffold of the BPTI host. The enantioselective recognition is even observable by the naked eye due to a specific exciplex-type emission originating from the interacting homochiral π-scaffolds of electron-rich [6]helicene and electron-poor BPTI.
Keywords: Binding Studies; Chirality; Dyes/Pigments; Enantioselectivity; Molecular Recognition.
© 2022 The Authors. Angewandte Chemie International Edition published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Kubik S., Supramolecular Chemistry, De Gruyter, Berlin/Boston, 2021.
-
- None
-
- Chao Y., Cram D. J., J. Am. Chem. Soc. 1976, 98, 1015–1017; for a recent review, see:
-
- Zhang Z., Shao Y., Tang J., Jiang J., Wang L., Li S., Green Synth. Catal. 2021, 2, 156–164.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

