Raman-Deuterium Isotope Probing and Metagenomics Reveal the Drought Tolerance of the Soil Microbiome and Its Promotion of Plant Growth
- PMID: 35103487
- PMCID: PMC8805637
- DOI: 10.1128/msystems.01249-21
Raman-Deuterium Isotope Probing and Metagenomics Reveal the Drought Tolerance of the Soil Microbiome and Its Promotion of Plant Growth
Abstract
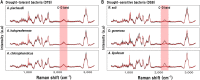
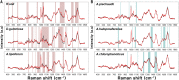
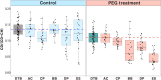
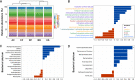
Drought has become a major agricultural threat leading crop yield loss. Although a few species of rhizobacteria have the ability to promote plant growth under drought, the drought tolerance of the soil microbiome and its relationship with the promotion of plant growth under drought are scarcely studied. This study aimed to develop a novel approach for assessing drought tolerance in agricultural land by quantitatively measuring microbial phenotypes using stable isotopes and Raman spectroscopy. Raman spectroscopy with deuterium isotope probing was used to identify the Raman signatures of drought effects from drought-tolerant bacteria. Counting drought-tolerant cells by applying these phenotypic properties to agricultural samples revealed that 0% to 52.2% of all measured single cells had drought-tolerant properties, depending on the soil sample. The proportions of drought-tolerant cells in each soil type showed similar tendencies to the numbers of revived pea plants cultivated under drought. The phenotype of the soil microbiome and plant behavior under drought conditions therefore appeared to be highly related. Studying metagenomics suggested that there was a reliable link between the phenotype and genotype of the soil microbiome that could explain mechanisms that promote plant growth in drought. In particular, the proportion of drought-tolerant cells was highly correlated with genes encoding phytohormone production, including tryptophan synthase and isopentenyl-diphosphate delta-isomerase; these enzymes are known to alleviate drought stress. Raman spectroscopy with deuterium isotope probing shows high potential as an alternative technology for quantitatively assessing drought tolerance through phenotypic analysis of the soil microbiome. IMPORTANCE Soil microbiome has played a critical role in the plant survival during drought. However, the drought tolerance of soil microbiome and its ability to promote plant growth under drought is still scarcely studied. In this study, we identified the Raman signature (i.e., phenotype) of drought effects from drought-tolerant bacteria in agricultural soil samples using Raman-deuterium isotope probing (Raman-DIP). Moreover, the number of drought-tolerant cells measured by Raman-DIP was highly related to the survival rate of plant cultivation under drought and the abundance of genes encoding phytohormone production alleviating drought stress in plant. These results suggest Raman-DIP is a promising technology for measuring drought tolerance of soil microbiome. This result give us important insight into further studies of a reliable link between phenotype and genotype of soil microbiome for future plant-bacteria interaction research.
Keywords: Raman-DIP; drought; drought tolerance; metagenomics; phenotype; soil microbiome.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Patterns in the Microbial Community of Salt-Tolerant Plants and the Functional Genes Associated with Salt Stress Alleviation.Microbiol Spectr. 2021 Oct 31;9(2):e0076721. doi: 10.1128/Spectrum.00767-21. Epub 2021 Oct 27. Microbiol Spectr. 2021. PMID: 34704793 Free PMC article.
-
Beneficial Root-Associated Microbiome during Drought and Flooding Stress in Plants.Pak J Biol Sci. 2023 Apr;26(5):287-299. doi: 10.3923/pjbs.2023.287.299. Pak J Biol Sci. 2023. PMID: 37859559 Review.
-
Microbial Drivers of Plant Performance during Drought Depend upon Community Composition and the Greater Soil Environment.Microbiol Spectr. 2023 Mar 21;11(2):e0147622. doi: 10.1128/spectrum.01476-22. Online ahead of print. Microbiol Spectr. 2023. PMID: 36943043 Free PMC article.
-
Manipulating rhizosphere microorganisms to improve crop yield in saline-alkali soil: a study on soybean growth and development.Front Microbiol. 2023 Sep 20;14:1233351. doi: 10.3389/fmicb.2023.1233351. eCollection 2023. Front Microbiol. 2023. PMID: 37799597 Free PMC article.
-
Mitigating abiotic stress: microbiome engineering for improving agricultural production and environmental sustainability.Planta. 2022 Sep 20;256(5):85. doi: 10.1007/s00425-022-03997-x. Planta. 2022. PMID: 36125564 Review.
Cited by
-
Tracking deuterium uptake in hydroponically grown maize roots using correlative helium ion microscopy and Raman micro-spectroscopy.Plant Methods. 2023 Jul 14;19(1):71. doi: 10.1186/s13007-023-01040-y. Plant Methods. 2023. PMID: 37452400 Free PMC article.
-
Interpretable machine learning decodes soil microbiome's response to drought stress.Environ Microbiome. 2024 May 29;19(1):35. doi: 10.1186/s40793-024-00578-1. Environ Microbiome. 2024. PMID: 38812054 Free PMC article.
-
Single-cell Raman and functional gene analyses reveal microbial P solubilization in agriculture waste-modified soils.mLife. 2023 Jun 14;2(2):190-200. doi: 10.1002/mlf2.12053. eCollection 2023 Jun. mLife. 2023. PMID: 38817623 Free PMC article.
-
Raman Microspectroscopy to Trace the Incorporation of Deuterium from Labeled (Micro)Plastics into Microbial Cells.Anal Chem. 2025 Mar 4;97(8):4440-4451. doi: 10.1021/acs.analchem.4c05827. Epub 2025 Feb 10. Anal Chem. 2025. PMID: 39929209 Free PMC article.
-
Transcriptional Response and Plant Growth Promoting Activity of Pseudomonas fluorescens DR397 under Drought Stress Conditions.Microbiol Spectr. 2022 Aug 31;10(4):e0097922. doi: 10.1128/spectrum.00979-22. Epub 2022 Jul 12. Microbiol Spectr. 2022. PMID: 35863006 Free PMC article.
References
-
- Xu L, Dong Z, Chiniquy D, Pierroz G, Deng S, Gao C, Diamond S, Simmons T, Wipf HM-L, Caddell D, Varoquaux N, Madera MA, Hutmacher R, Deutschbauer A, Dahlberg JA, Guerinot ML, Purdom E, Banfield JF, Taylor JW, Lemaux PG, Coleman-Derr D. 2021. Genome-resolved metagenomics reveals role of iron metabolism in drought-induced rhizosphere microbiome dynamics. Nat Commun 12:1–17. doi:10.1038/s41467-021-23553-7. - DOI - PMC - PubMed
-
- Vardharajula S, Zulfikar Ali S, Grover M, Reddy G, Bandi V. 2011. Drought-tolerant plant growth promoting Bacillus spp.: effect on growth, osmolytes, and antioxidant status of maize under drought stress. J Plant Interactions 6:1–14. doi:10.1080/17429145.2010.535178. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
