A single full-length VAR2CSA ectodomain variant purifies broadly neutralizing antibodies against placental malaria isolates
- PMID: 35103596
- PMCID: PMC8959597
- DOI: 10.7554/eLife.76264
A single full-length VAR2CSA ectodomain variant purifies broadly neutralizing antibodies against placental malaria isolates
Abstract
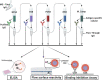
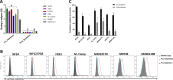
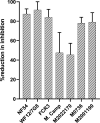
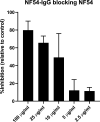
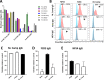
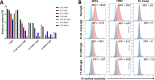
Placental malaria (PM) is a deadly syndrome most frequent and severe in first pregnancies. PM results from accumulation of Plasmodium falciparum-infected erythrocytes (IE) that express the surface antigen VAR2CSA and bind to chondroitin sulfate A (CSA) in the placenta. Women become PM-resistant over successive pregnancies as they develop anti-adhesion and anti-VAR2CSA antibodies, supporting VAR2CSA as the leading PM-vaccine candidate. However, the first VAR2CSA subunit vaccines failed to induce broadly neutralizing antibody and it is known that naturally acquired antibodies target both variant-specific and conserved epitopes. It is crucial to determine whether effective vaccines will require incorporation of many or only a single VAR2CSA variants. Here, IgG from multigravidae was sequentially purified on five full-length VAR2CSA ectodomain variants, thereby depleting IgG reactivity to each. The five VAR2CSA variants purified ~0.7% of total IgG and yielded both strain-transcending and strain-specific reactivity to VAR2CSA and IE-surface antigen. In two independent antibody purification/depletion experiments with permutated order of VAR2CSA variants, IgG purified on the first VAR2CSA antigen displayed broad cross-reactivity to both recombinant and native VAR2CSA variants, and inhibited binding of all isolates to CSA. IgG remaining after depletion on all variants showed significantly reduced binding-inhibition activity compared to initial total IgG. These findings demonstrate that a single VAR2CSA ectodomain variant displays conserved epitopes that are targeted by neutralizing (or binding-inhibitory) antibodies shared by multiple parasite strains, including maternal isolates. This suggests that a broadly effective PM-vaccine can be achieved with a limited number of VAR2CSA variants.
Trial registration: ClinicalTrials.gov NCT01168271.
Keywords: P. falciparum; VAR2CSA; blocking antibody; immunology; infectious disease; inflammation; malaria; microbiology; pregnancy; vaccine; variant.
Plain language summary
Contracting malaria during pregnancy – especially a first pregnancy – can lead to a severe, placental form of the disease that is often fatal. Red blood cells infected with the malaria parasite Plasmodium falciparum display a protein, VAR2CSA, which can recognize and bind CSA molecules present on placental cells and in placental blood spaces. This leads to the infected blood cells accumulating in the placenta and inducing harmful inflammation. Having been exposed to the parasite in prior pregnancies generates antibodies that target VAR2CSA, stopping the infected blood cells from latching onto placental CSA or tagging them for immune destruction. Overall, this makes placental malaria less severe in following pregnancies, and suggests that vaccines could be developed based on VAR2CSA. However, this protein has regions that can vary in structure, meaning that P. falciparaum can generate many VAR2CSA variants. Individuals exposed to the parasite naturally generate antibodies that block a wide array of variants from attaching to CSA. In contrast, first-generation vaccines based on VAR2CSA fragments have only induced variant-specific antibodies, therefore offering limited protection against infection. As a response, Doritchamou et al. set out to find VAR2CSA structures that could be recognized by antibodies targeting an array of variants. Blood was obtained from women who had had multiple pregnancies and were immune to malaria. Their plasma was passed over five different large VAR2CSA variants in order to isolate and purify antibodies that attached to these structures. Doritchamou et al. found that antibodies binding to individual VAR2CSA structures could also recognise a wide array of VAR2CSA variants and blocked all tested parasites from sticking to CSA. While further research is needed, these findings highlight antibodies that cross-react to diverse VAR2CSA variants and could be used to design more effective vaccines targeting placental malaria.
Conflict of interest statement
JD, JR, BJ, AM, AD, MF, PD No competing interests declared
Figures




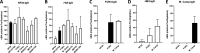
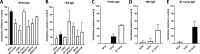
Comment in
-
Tackling variants with antibodies.Elife. 2022 Mar 28;11:e77751. doi: 10.7554/eLife.77751. Elife. 2022. PMID: 35344481 Free PMC article.
Similar articles
-
VAR2CSA Domain-Specific Analysis of Naturally Acquired Functional Antibodies to Plasmodium falciparum Placental Malaria.J Infect Dis. 2016 Aug 15;214(4):577-86. doi: 10.1093/infdis/jiw197. Epub 2016 May 18. J Infect Dis. 2016. PMID: 27190180 Free PMC article.
-
Differential induction of functional IgG using the Plasmodium falciparum placental malaria vaccine candidate VAR2CSA.PLoS One. 2011 Mar 25;6(3):e17942. doi: 10.1371/journal.pone.0017942. PLoS One. 2011. PMID: 21464946 Free PMC article.
-
A conformational epitope in placental malaria vaccine antigen VAR2CSA: What does it teach us?PLoS Pathog. 2023 May 25;19(5):e1011370. doi: 10.1371/journal.ppat.1011370. eCollection 2023 May. PLoS Pathog. 2023. PMID: 37228009 Free PMC article.
-
Designing a VAR2CSA-based vaccine to prevent placental malaria.Vaccine. 2015 Dec 22;33(52):7483-8. doi: 10.1016/j.vaccine.2015.10.011. Epub 2015 Nov 26. Vaccine. 2015. PMID: 26469717 Free PMC article. Review.
-
Progress and Insights Toward an Effective Placental Malaria Vaccine.Front Immunol. 2021 Feb 25;12:634508. doi: 10.3389/fimmu.2021.634508. eCollection 2021. Front Immunol. 2021. PMID: 33717176 Free PMC article. Review.
Cited by
-
A machine learning framework to identify complex physicochemical features of B cell epitopes.Res Sq [Preprint]. 2025 Apr 18:rs.3.rs-6255613. doi: 10.21203/rs.3.rs-6255613/v1. Res Sq. 2025. PMID: 40321766 Free PMC article. Preprint.
-
Analysis of allelic cross-reactivity of monoclonal IgG antibodies by a multiplexed reverse FluoroSpot assay.Elife. 2022 Jul 15;11:e79245. doi: 10.7554/eLife.79245. Elife. 2022. PMID: 35838346 Free PMC article.
-
Structure-guided design of VAR2CSA-based immunogens and a cocktail strategy for a placental malaria vaccine.PLoS Pathog. 2024 Mar 4;20(3):e1011879. doi: 10.1371/journal.ppat.1011879. eCollection 2024 Mar. PLoS Pathog. 2024. PMID: 38437239 Free PMC article.
-
Detection of naturally acquired, strain-transcending antibodies against rosetting Plasmodium falciparum strains in humans.Infect Immun. 2024 Jul 11;92(7):e0001524. doi: 10.1128/iai.00015-24. Epub 2024 Jun 6. Infect Immun. 2024. PMID: 38842304 Free PMC article.
-
Tackling variants with antibodies.Elife. 2022 Mar 28;11:e77751. doi: 10.7554/eLife.77751. Elife. 2022. PMID: 35344481 Free PMC article.
References
-
- Avril M, Hathaway MJ, Srivastava A, Dechavanne S, Hommel M, Beeson JG, Smith JD, Gamain B. Antibodies to a full-length VAR2CSA immunogen are broadly strain-transcendent but do not cross-inhibit different placental-type parasite isolates. PLOS ONE. 2011;6:e16622. doi: 10.1371/journal.pone.0016622. - DOI - PMC - PubMed
-
- Barfod L, Bernasconi NL, Dahlbäck M, Jarrossay D, Andersen PH, Salanti A, Ofori MF, Turner L, Resende M, Nielsen MA, Theander TG, Sallusto F, Lanzavecchia A, Hviid L. Human pregnancy-associated malaria-specific B cells target polymorphic, conformational epitopes in VAR2CSA. Molecular Microbiology. 2007;63:335–347. doi: 10.1111/j.1365-2958.2006.05503.x. - DOI - PMC - PubMed
-
- Benavente ED, Oresegun DR, de Sessions PF, Walker EM, Roper C, Dombrowski JG, de Souza RM, Marinho CRF, Sutherland CJ, Hibberd ML, Mohareb F, Baker DA, Clark TG, Campino S. Global genetic diversity of var2csa in Plasmodium falciparum with implications for malaria in pregnancy and vaccine development. Scientific Reports. 2018;8:15429. doi: 10.1038/s41598-018-33767-3. - DOI - PMC - PubMed
-
- Bertin GI, Sabbagh A, Guillonneau F, Jafari-Guemouri S, Ezinmegnon S, Federici C, Hounkpatin B, Fievet N, Deloron P. Differential protein expression profiles between Plasmodium falciparum parasites isolated from subjects presenting with pregnancy-associated malaria and uncomplicated malaria in Benin. The Journal of Infectious Diseases. 2013;208:1987–1997. doi: 10.1093/infdis/jit377. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

