Synthetic Neoglycoconjugates of Hepta- and Nonamannoside Ligands for Eliciting Oligomannose-Specific HIV-1-Neutralizing Antibodies
- PMID: 35104013
- PMCID: PMC9108342
- DOI: 10.1002/cbic.202200061
Synthetic Neoglycoconjugates of Hepta- and Nonamannoside Ligands for Eliciting Oligomannose-Specific HIV-1-Neutralizing Antibodies
Abstract
Oligomannose-type glycans on the spike protein of HIV-1 constitute relevant epitopes to elicit broadly neutralizing antibodies (bnAbs). Herein we describe an improved synthesis of α- and β-linked hepta- and nonamannosyl ligands that were subsequently converted into BSA and CRM197 neoglycoconjugates. We assembled the ligands from anomeric 3-azidopropyl spacer glycosides from select 3-O-protected thiocresyl mannoside donors. Chain extensions were achieved using [4+3] or [4+5] block synthesis of thiocresyl and trichloroacetimidate glycosyl donors. Subsequent global deprotection generated the 3-aminopropyl oligosaccharide ligands. ELISA binding data obtained with the β-anomeric hepta- and nonamannosyl conjugates with a selection of HIV-1 bnAbs showed comparable binding of both mannosyl ligands by Fab fragments yet lesser binding of the nonasaccharide conjugate by the corresponding IgG antibodies. These results support previous observations that a complete Man9 structure might not be the preferred antigenic binding motif for some oligomannose-specific antibodies, and have implications for glycoside designs to elicit oligomannose-targeted HIV-1-neutralizing antibodies.
Keywords: HIV/AIDS; glycoconjugates; glycosylation; oligomannosides; synthesis.
© 2022 The Authors. ChemBioChem published by Wiley-VCH GmbH.
Figures
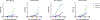
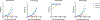
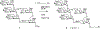

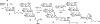




References
-
- Walker LM, Simek MD, Priddy F, Gach JS, Wagner D, Zwick MB, Phogat SK, Burton DR, PLoS Pathog 2010, 6, e1001028 (DOI: 10.1371/journal.ppat.1001028); - DOI - PMC - PubMed
- Jacob RA, Moyo T, Schomaker M, Abrahams F, Pujola BG, Dorfman JR, J. Virol. 2015, 89, 5264–5275; - PMC - PubMed
- Ditse Z, Muenchhoff M, Adland E, Joosteg P, Goulder P, Moore PL, Morris L, J. Virol. 2018, 92, e00878 (DOI: 10.1128/jvi.00878-18); - DOI - PMC - PubMed
- Tomaras GD, Binley JM, Gray ES, Crooks ET, Osawa K, Moore PL, Tumba N, Tong T, Shen X, Yates NL, Decker J, Wibmer CK, Gao F, Alam SM, Easterbrook P, Karim SA, Kamanga G, Crump JA, Cohen M, Shaw GM, Mascola JR, Haynes BF, Montefiori DC, Morris L, J. Virol. 2011, 85, 11502–11519; - PMC - PubMed
- Landais E, Huang X, Havenar-Daughton C, Murrell B, Price MA, Wickramasinghe L, Ramos A, Bian CB, Simek M, Allen S, Karita E, Kilembe W, Lakhi S, Inambao M, Kamali A, Sanders EJ, Anzala O, Edward V, Bekker L-G, Tang J, Gilmour J, Kosakovsky-Pond SL, Phung P, Wrin T, Crotty S, Godzik A, Poignard P. PLOS Path. 2016, 12, e 1005369 (DOI: 10.1371/journal.ppat.1005369); - DOI - PMC - PubMed
- Moore PL, Gray ES, Wibmer CK, Bhiman JN, Nonyane M, Sheward DJ, Hermanus T, Bajimaya S, Tumba NL, Abrahams M-R, Lambson BE, Ranchobe N, Ping L, Ngandu N, Karim QA, Karim SA, Swanstrom RI, Seaman MS, Williamson C, Morris L, Nat Med 2012, 18, 1688–1692. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

