Accumulation of meningeal lymphocytes correlates with white matter lesion activity in progressive multiple sclerosis
- PMID: 35104246
- PMCID: PMC8983127
- DOI: 10.1172/jci.insight.151683
Accumulation of meningeal lymphocytes correlates with white matter lesion activity in progressive multiple sclerosis
Abstract
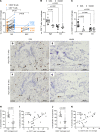
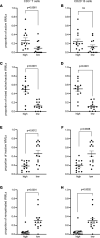
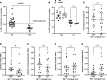
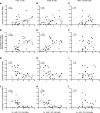
Subpial cortical demyelination is an important component of multiple sclerosis (MS) pathology contributing to disease progression, yet mechanism(s) underlying its development remain unclear. Compartmentalized inflammation involving the meninges may drive this type of injury. Given recent findings identifying substantial white matter (WM) lesion activity in patients with progressive MS, elucidating whether and how WM lesional activity relates to meningeal inflammation and subpial cortical injury is of interest. Using postmortem FFPE tissue blocks (range, 5-72 blocks; median, 30 blocks) for each of 27 patients with progressive MS, we assessed the relationship between meningeal inflammation, the extent of subpial cortical demyelination, and the state of subcortical WM lesional activity. Meningeal accumulations of T cells and B cells, but not myeloid cells, were spatially adjacent to subpial cortical lesions, and greater immune cell accumulation was associated with larger subpial lesion areas. Patients with a higher extent of meningeal inflammation harbored a greater proportion of active and mixed active/inactive WM lesions and an overall lower proportion of inactive and remyelinated WM lesions. Our findings support the involvement of meningeal lymphocytes in subpial cortical injury and point to a potential link between inflammatory subpial cortical demyelination and pathological mechanisms occurring in the subcortical WM.
Keywords: Adaptive immunity; Demyelinating disorders; Immunology; Multiple sclerosis.
Conflict of interest statement
Figures
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

