Obesity and risk of female reproductive conditions: A Mendelian randomisation study
- PMID: 35104295
- PMCID: PMC8806071
- DOI: 10.1371/journal.pmed.1003679
Obesity and risk of female reproductive conditions: A Mendelian randomisation study
Erratum in
-
Correction: Obesity and risk of female reproductive conditions: A Mendelian randomisation study.PLoS Med. 2022 Sep 2;19(9):e1004095. doi: 10.1371/journal.pmed.1004095. eCollection 2022 Sep. PLoS Med. 2022. PMID: 36054878 Free PMC article.
Abstract
Background: Obesity is observationally associated with altered risk of many female reproductive conditions. These include polycystic ovary syndrome (PCOS), abnormal uterine bleeding, endometriosis, infertility, and pregnancy-related disorders. However, the roles and mechanisms of obesity in the aetiology of reproductive disorders remain unclear. Thus, we aimed to estimate observational and genetically predicted causal associations between obesity, metabolic hormones, and female reproductive disorders.
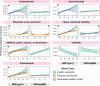
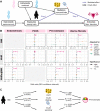
Methods and findings: Logistic regression, generalised additive models, and Mendelian randomisation (MR) (2-sample, non-linear, and multivariable) were applied to obesity and reproductive disease data on up to 257,193 women of European ancestry in UK Biobank and publicly available genome-wide association studies (GWASs). Body mass index (BMI), waist-to-hip ratio (WHR), and WHR adjusted for BMI were observationally (odds ratios [ORs] = 1.02-1.87 per 1-SD increase in obesity trait) and genetically (ORs = 1.06-2.09) associated with uterine fibroids (UF), PCOS, heavy menstrual bleeding (HMB), and pre-eclampsia. Genetically predicted visceral adipose tissue (VAT) mass was associated with the development of HMB (OR [95% CI] per 1-kg increase in predicted VAT mass = 1.32 [1.06-1.64], P = 0.0130), PCOS (OR [95% CI] = 1.15 [1.08-1.23], P = 3.24 × 10-05), and pre-eclampsia (OR [95% CI] = 3.08 [1.98-4.79], P = 6.65 × 10-07). Increased waist circumference posed a higher genetic risk (ORs = 1.16-1.93) for the development of these disorders and UF than did increased hip circumference (ORs = 1.06-1.10). Leptin, fasting insulin, and insulin resistance each mediated between 20% and 50% of the total genetically predicted association of obesity with pre-eclampsia. Reproductive conditions clustered based on shared genetic components of their aetiological relationships with obesity. This study was limited in power by the low prevalence of female reproductive conditions among women in the UK Biobank, with little information on pre-diagnostic anthropometric traits, and by the susceptibility of MR estimates to genetic pleiotropy.
Conclusions: We found that common indices of overall and central obesity were associated with increased risks of reproductive disorders to heterogenous extents in a systematic, large-scale genetics-based analysis of the aetiological relationships between obesity and female reproductive conditions. Our results suggest the utility of exploring the mechanisms mediating the causal associations of overweight and obesity with gynaecological health to identify targets for disease prevention and treatment.
Conflict of interest statement
I have read the journal’s policy and the authors of this manuscript have the following competing interests: C.M.B. reports grants from Bayer AG, AbbVie Inc, Volition Rx, MDNA Life Sciences, Roche Diagnostics Inc., and consultancy for Myovant. He is a member of the independent data monitoring board at ObsEva; I.G. reports grants from Bayer AG; K.T.Z. reports grants from Bayer AG, AbbVie Inc, Volition Rx, MDNA Life Sciences, Roche Diagnostics Inc, and non-financial scientific collaboration with Population Diagnostics Ltd, outside the submitted work; M.V.H. has consulted for Boehringer Ingelheim, and in adherence to the University of Oxford’s Clinical Trial Service Unit & Epidemiological Studies Unit (CSTU) staff policy, did not accept personal honoraria or other payments from pharmaceutical companies; C.M.L. reports grants from Bayer AG and Novo Nordisk and has a partner who works at Vertex; no other relationships or activities that could appear to have influenced the submitted work. All disclosed competing interests are outside the submitted work.
Figures



References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

